1. Background
Cisplatin (CPN) is widely used for the management of various malignant tumors. However, CPN impairs the male reproductive system and can cause infertility in men undergoing chemotherapy (1-3). Cisplatin leads to impaired steroidogenesis, germ cell apoptosis, and changes in testicular histology (1, 4, 5). Previous studies have shown that CPN disrupts autophagy in the testes (6, 7).
Autophagy-related genes are highly expressed in spermatozoa, suggesting that autophagy regulates sperm quality. LC3, a biomarker of autophagy, is activated during sperm capacitation and the acrosome reaction (8-10).
P62 is an autophagy receptor that suppresses autophagy. This multifunctional protein integrates death and survival signals, modulating apoptosis and autophagy to maintain cellular homeostasis (11).
Black seed (Nigella sativa) is widely used in traditional medicine in Iran. Thymoquinone (TQN), derived from Nigella sativa, has shown numerous pharmacological and biological effects (12-15).
Thymoquinone has been found to enhance spermatogenesis and improve testicular damage caused by ischemia-reperfusion injury (16) and testicular torsion (17). Moreover, TQN protects against testicular damage induced by bleomycin (18), cyclophosphamide, and methotrexate through anti-inflammatory, anti-apoptotic, and antioxidant mechanisms (18-20).
2. Objectives
Given the important role of autophagy in spermatogenesis and spermiogenesis, this study aimed to investigate the effects of TQN and CPN on autophagy in mouse testicular tissue by evaluating the expression of p62 and LC3-II.
3. Methods
3.1. Animals
Thirty-two NMRI mice (6 - 8 weeks old, 25 - 30 g) were kept under standard conditions (12-hour dark/light cycles, 20 - 25°C). The animals were divided into the following groups:
- Control: Intraperitoneal (i.p.) injection of normal saline for 35 days.
- CPN: i.p. injection of CPN (7 mg/kg) on days 30 - 35 (21).
- TQN10: i.p. injection of TQN (10 mg/kg) for 35 days (22).
- TQN + CPN: i.p. injection of TQN for 35 days and CPN on days 30 - 35.
At the end of the experiment, the right testicles were preserved in Bouin's fixative for histomorphometric assessment, and the left testicles were stored at -70°C for evaluating autophagy.
3.2. Histology
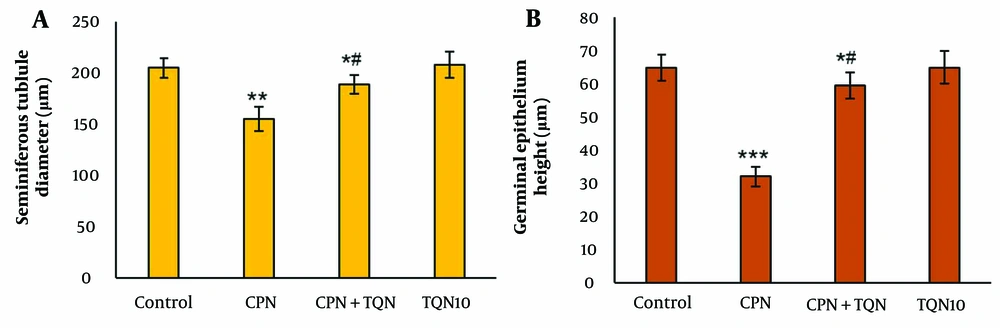
After tissue processing, the slides were prepared and stained with Hematoxylin and Eosin. Two researchers, blinded to group allocation, independently analyzed the slides. Seminiferous tubule diameters and germinal epithelium height were calculated using Motic software. One hundred tubules per animal were evaluated.
Testicular injury was scored using the Cosentino grading system (23), which grades testicular damage from I to IV:
- Grade I: Normal testis tissue.
- Grade II: Closely packed seminiferous tubules with non-cohesive germinal cells.
- Grade III: Sloughing and shrinking of the seminiferous epithelium.
- Grade IV: Closely packed seminiferous tubules with coagulated necrosis.
2.3. Real-time PCR
The RNA from the testicles was isolated using a RNeasy Mini kit (Qiagen) and converted to cDNA. A PCR reaction (25 μL) containing cDNA, primers (Table 1), DEPC water, and SYBR Green (Qiagen) was prepared. The relative expression of p62 was normalized to GAPDH using REST software.
| Genes | Forward | Reverse |
|---|---|---|
| p62 | GCTCAGGAGGAGACGATGAC | AGAAACCCATGGACAGCATC |
| GAPDH | GCTGGACATTGGACTTCCTC | ACCACTGTGACCTGCTCCA |
2.4. Protein Levels of LC3-II
The levels of LC3-II were measured in the culture supernatants using a commercially available ELISA kit (Abcam, USA) according to the manufacturer's instructions. Both primary and secondary antibodies were added to the tissue lysates. The protein levels were quantified using an ELISA reader (BioTek, USA) by measuring the absorbance at 450 nm.
2.5. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed using SPSS (version 21.0), followed by a post hoc test (LSD or Kruskal-Wallis). A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Histology
The mean Cosentino score in the CPN-treated mice was significantly reduced compared to the control group. Thymoquinone treatment was able to reverse the mean Cosentino score (Table 2).
| Groups | Grade I | Grade II | Grade III | Grade IV | Mean ± SD |
|---|---|---|---|---|---|
| Control (n = 8) | 8 | - | - | - | 1.0 ± 0.0 |
| CPN (n = 8) | - | - | 5 | 3 | 3. 8 ± 0.4** |
| CPN+TQN (n = 8) | 1 | 3 | 2 | 2 | 1.6 ± 0.2*# |
| TQN10 (n = 8) | 8 | - | - | - | 1.0 ± 0.0 |
Abbreviations: CPN, cisplatin; TQN, thymoquinone.
a *#: P < 0.05; **: P < 0.01.
There were no significant changes in the morphometric parameters in the TQN10 group. However, in the CPN group, morphometric parameters were significantly decreased compared to the control (P < 0.01). In the TQN + CPN group, morphometric parameters were significantly higher than those in the CPN group (P < 0.05) (Figure 1).
4.2. Autophagy Assessments
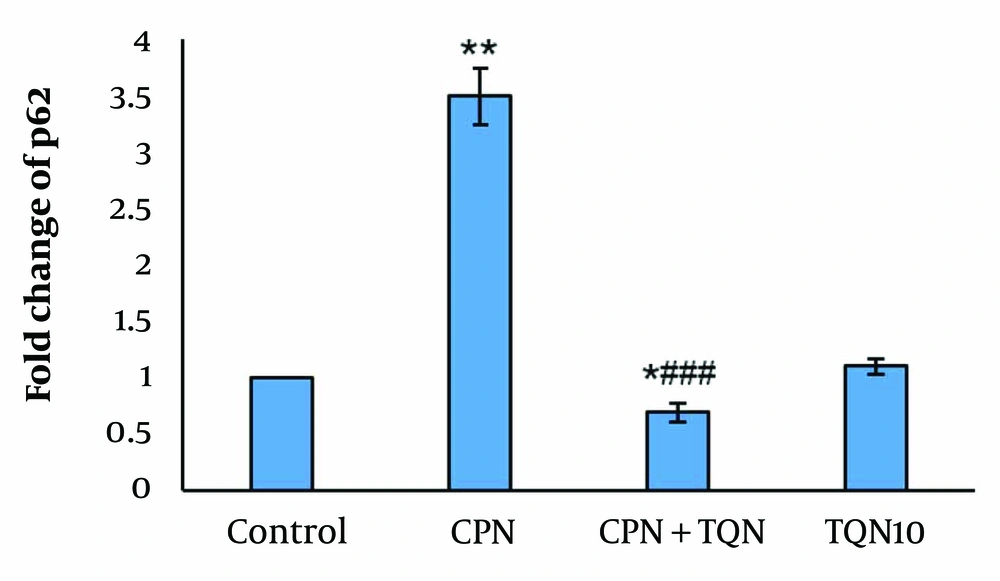
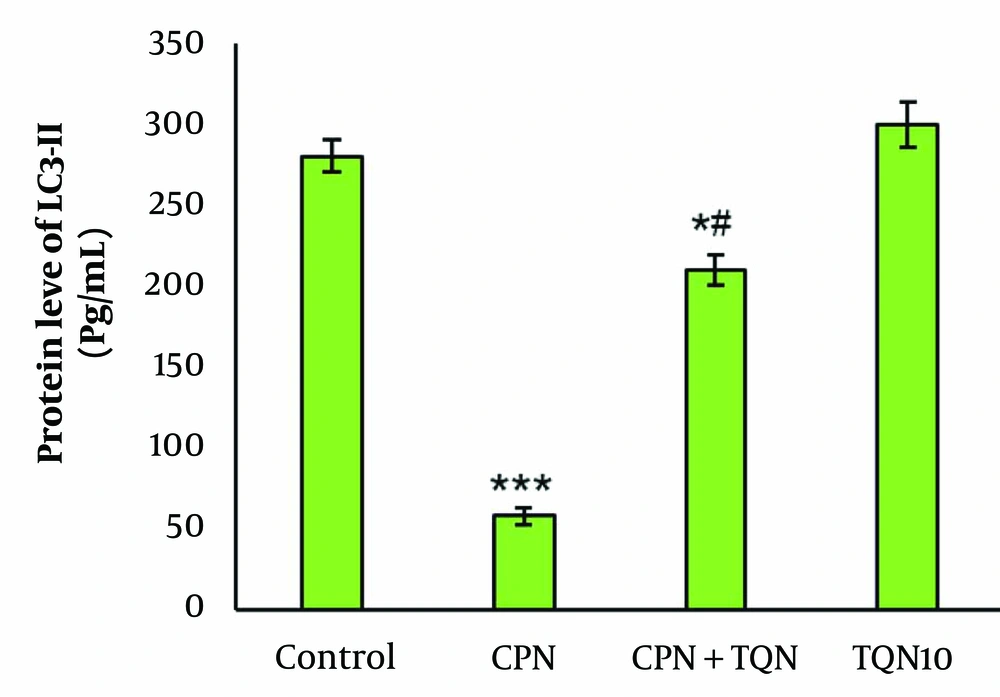
There were no significant changes in p62 gene expression or LC3-II protein levels in the TQN10 group. In the CPN group, p62 expression was significantly upregulated, while LC3-II protein levels were downregulated (P < 0.01). In the TQN + CPN group, LC3-II levels were markedly increased compared to the CPN-treated mice, and p62 expression was significantly reduced compared to the CPN group (Figures 2 and 3).
5. Discussion
In this study, CPN induced impaired testicular tissue, increased expression of the p62 gene, and reduced expression of the LC3-II protein. The administration of TQN significantly reversed these effects.
The decrease in germinal epithelium height and seminiferous tubule diameter indicates the toxic impact of CPN. The increased Cosentino score further confirms the testicular toxicity caused by CPN.
The decline in morphometric parameters and the rise in the Cosentino score may suggest an apoptotic effect of CPN. A previous study showed that CPN reduced sperm quality, decreased the diameter of seminiferous tubules, and induced apoptosis in rats (24). In our earlier research, CPN increased Bax (a pro-apoptotic factor) in the mouse testis (25). The observed improvement in histological changes suggests that TQN protects against CPN-induced testicular damage. In another study, TQN ameliorated histological changes in testicular tissue following ischemia (17).
The improvement in histomorphometric parameters and reduction in the Cosentino score in this study may indicate the anti-apoptotic effects of TQN. Anti-apoptotic effects of TQN on damaged testes have been reported previously (26-28). Thymoquinone was shown to reduce apoptosis in the testicles of doxorubicin-intoxicated animals (29). In another study, TQN significantly decreased apoptosis in varicocele-induced rats (26).
In this study, the increased fold change of the p62 gene and the reduced protein level of LC3-II suggest that CPN suppresses autophagy. Therefore, CPN may impair spermatogenesis by inhibiting autophagy.
In our study, TQN pretreatment elevated LC3-II protein levels while decreasing the expression of p62 in the CPN group. These findings suggest that TQN promotes autophagy in CPN-induced testicular damage. Liao et al. showed that CPN activated autophagy in renal damaged tissue through a marked increase in p62 (30).
Interestingly, we found that CPN-induced testicular damage was associated with autophagy inhibition. In contrast, TQN decreased p62 expression, increased LC3-II protein levels, and improved testicular histology.
Liu et al. demonstrated that TQN reduces doxorubicin-induced apoptosis in H9c2 cardiomyocytes by inducing autophagy (31). In another study, TQN induced autophagy in renal cancer cells (32). Additionally, TQN attenuates lipid accumulation by activating autophagy in nonalcoholic fatty liver disease (33).
5.1. Conclusions
Our results demonstrated that TQN activated autophagy by suppressing p62 expression. These data suggest that TQN could be a promising adjunct therapy against CPN-induced testicular toxicity in future anticancer clinical practice.