1. Background
Scorpions, which have existed for over 400 million years, produce venom rich in bioactive compounds, including peptides with diverse therapeutic potential (1). These peptides, categorized into disulfide-bridged and non-disulfide-bridged types, exhibit activities such as ion channel modulation and immune regulation (2). Scorpion venoms have been extensively studied for their anti-cancer properties, with peptides demonstrating apoptosis induction, metastasis inhibition, and immune response modulation (3). For instance, peptides such as neopaladins from Tityus discrepans selectively induce apoptosis in SKBR3 breast cancer cells through mechanisms involving FasL, MAP kinases, and p53 (3, 4). The immune system plays a crucial role in tumor suppression, with strategies like immunotherapy targeting residual cancer cells by enhancing immune responses (5, 6). Scorpion venom peptides have shown the ability to modulate immune responses by stimulating cytokine and chemokine production, recruiting neutrophils and macrophages, and enhancing phagocytosis (7-9). Specific peptides, such as parabutoporin, activate G-protein signaling pathways, promoting chemotaxis and inflammation (10). Similarly, venom from Tityus serrulatus increases cytokine levels in macrophages, highlighting its immunomodulatory effects (11, 12).
Among scorpion venom peptides, the HL-7 and HL-10 peptides from Hemiscorpius lepturus have demonstrated apoptosis-inducing properties, particularly in HeLa cells, through the intrinsic mitochondrial pathway (13-15). These peptides upregulate pro-apoptotic genes (e.g., bax, Cyt c, p53) while downregulating anti-apoptotic genes (e.g., bcl-2), thereby initiating programmed cell death (15). However, the specific effects of HL-10 on SiHa cervical cancer cells, particularly its influence on apoptosis and cytokine regulation, remain unexplored. Cervical cancer is a significant global health issue, with SiHa cells serving as a model for studying HPV-related malignancies. Current therapies like chemotherapy have limitations, including toxicity and resistance, highlighting the need for novel therapeutic agents (16). This study investigates the anti-cancer potential of the HL-10 peptide in SiHa cervical cancer cells, focusing on its ability to induce apoptosis and modulate immune responses.
2. Objectives
By assessing gene expression, caspase activity, and cytokine levels, this research aims to elucidate the mechanisms underlying HL-10's therapeutic effects.
3. Methods
3.1. Cell Culture Process
In this experiment, the SiHa cervical cancer cell line was acquired from the Pasteur Institute Cell Bank of Tehran, Iran. The cells were cultured in RPMI-1640 medium, supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS). They were then incubated at 37°C in a controlled environment with 5% CO2 and 95% humidity. The culture medium was changed every 48 hours, and the cells were routinely passaged to maintain growth until they reached approximately 70% confluence.
3.2. Preparation of HL-10 Peptide and Carboplatin
The HL-10 peptide was synthesized by Custom Peptide Factory, China, ensuring a purity of > 95%. The peptide was dissolved in sterile phosphate-buffered saline (PBS). The solution was aliquoted into sterile microcentrifuge tubes and stored at -20°C to prevent degradation. Prior to use, the stock solution was thawed at room temperature and diluted to the desired concentrations using PBS.
Carboplatin (CAS 41575-94-4, Merck) was obtained as a powder and dissolved in sterile PBS. The solution was filter-sterilized using a 0.22 μm syringe filter, aliquoted into sterile tubes, and stored at 4°C. The solution was protected from light by wrapping the tubes in aluminum foil. Fresh working dilutions of carboplatin were prepared in PBS immediately before each experiment to ensure stability.
3.3. (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay
First, the cells were cultured at a density of 5 × 106 cells in a 24-well plate. Approximately two hours after cultivation, SiHa cells were exposed to the HL-10 peptide at doses ranging from 0 to 25 μM, and to carboplatin at concentrations ranging from 0 to 70 μM, for periods of 24 and 48 hours. Following the treatment, each well received 150 μL of DMSO (dimethyl sulfoxide) to dissolve the formazan crystals. The plates were then incubated with 100 μL of (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent for 4 hours. After incubation, the absorbance of the resulting formazan solution, indicative of viable cells, was measured at 540 nm using an ELISA reader (BioTek Epoch 2, Germany). The accompanying software (Gen5 Data Analysis Software) was used to record and analyze the optical density (OD) values. The percentage of cell viability was calculated relative to untreated control cells. Each treatment was performed in triplicate to ensure reproducibility (15).
3.4. RNA Extraction, cDNA Synthesis, and PCR
A total of 4 × 106 SiHa cells were exposed to the HL-10 peptide at 5 and 10 µM for 48 hours. For cDNA synthesis, 3 µL of total RNA, 1 µL of dT oligo primer, and 10 µL of deionized distilled water were incubated at 65°C for 5 minutes. Subsequently, 1 µL of reverse transcriptase enzyme, 2 µL of 10 mM dNTP, and 6 µL of 5X PCR buffer were added. The mixture was incubated at 25°C for 10 minutes, 50°C for 60 minutes, and 70°C for 10 minutes. RT-PCR was performed according to Parstous Company’s protocol. The reaction mixture (29 µL) contained 3 µL of 10X buffer, 0.2 µL of Taq DNA polymerase, 1 µL of cDNA, 0.5 µL of dNTP, 2 µL of primers, 1 µL of magnesium chloride, and 21.3 µL of water. The thermocycler was set for 30 cycles: 65°C for 30 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. Real-time PCR primers for target genes and GAPDH were used, with a 20 µL mixture including SYBR Green and ROX dye, as detailed in Table 1. Gene expression was assessed using real-time PCR.
| Genes | Primer Sequences: 5′ → 3′ |
|---|---|
| Bax | F-CCTGTGCACCAAGGTGCCGGAACT |
| R-CCACCCTGGTCTTGGATCCAGCCC | |
| GAPDH | F-AGCCAAAAGGGTCATCATC |
| R-TAAGCAGTTGGTGGTGCAGG | |
| Caspase 8 | F-AAGGGAGGCAAGCACAAGACTG |
| R-CTCCATCAGTGTATCCTCTCCC | |
| Caspase 3 | F-TAAGTTCTGAGTGTGACCGAGA |
| R-GCTCTGTCTGTAGGGAGGTAGG | |
| Caspase 9 | F-CGACATGATCGAGGATATTCAG |
| R-TGCCTCCCTCGAGTCTCA | |
| Bcl-2 | F-GATGTGATGCCTCTGCGAAG |
| R-CATGCTGATGTCTCTGGAATCT | |
| Cytochrome c | F-AAGGGAGGCAAGCACAAGACTG |
| R-CTCCATCAGTGTATCCTCTCCC | |
| P53 | F-AGTCTAGAGCCACCGTCCA |
| R-TCTGACGCACACCTATTGCAAGC |
3.5. Measuring the Activity of Caspases 9, 8, and 3
To measure the activity of caspases 9, 8, and 3, the Abcam colorimetric kit (Abcam, Cambridge, UK) was used. A total of 5 × 106 cells were treated with HL-10 peptide at concentrations of 5 μM and 10 μM for 48 hours. An untreated control group was also included for comparative analysis. After treatment, the cells were lysed in 50 μL of cell lysis buffer on ice for 30 minutes. The lysates were then centrifuged at 1000 rpm for 15 minutes to remove cellular debris. Protein concentrations in the assay were adjusted to 5 - 200 μg per 50 μL of buffer. To each well, 50 μL of a DTT/reaction buffer X2 mixture was added, followed by 5 μL of the caspase substrate specific to the caspase being studied. The plates were incubated at 37°C for 1 - 2 hours. Absorbance was then measured at 405 nm using an ELISA reader.
3.6. Animal Study
All experiments were conducted on female BALB/c mice (obtained from the Pasteur Institute, Karaj), aged 4 - 5 weeks and weighing between 12 and 17 grams. All in vivo procedures were approved by the Ethics Committee of Zabol University (IR.UOZ.REC.1401.012). The mice were housed under controlled conditions, including regulated light, temperature, and humidity, at the Animal Center of the Zabol University Faculty of Veterinary Medicine.
3.7. Induction of a Cervical Cancer Model
To induce a tumor model in mice, the SiHa cervical cancer cell line was used, and 100 µL of cell suspension in saline (1 × 106 cells) was injected subcutaneously into the right flank area of each mouse (16). After 10 days, 24 mice were divided into four groups (n = 6): A negative control (NC) group (healthy mice that did not undergo surgery and received only normal saline); a sham group (cancer-bearing mice that received only normal saline); a positive control (PC) group (cancer-bearing mice that received carboplatin at a dose of 5 mg/kg); and a treatment group (HL-10) (cancer-bearing mice that received the HL-10 peptide at the same dose as the positive control group). All treatments were administered intraperitoneally five times, every five days. Additionally, tumor volume was measured every five days in two dimensions—length and width—using a caliper, and the tumor volume was calculated using the formula (16):
V = length × width2 × 0.5
3.8. Tumor Measurement
For tumor measurements, the size of the tumors was assessed using a caliper to measure the longest diameter (length) and the shortest diameter (width). Tumor volume was calculated using the formula:
Volume = (length × width2)/2
Tumor growth was monitored at regular intervals, typically every 2 - 3 days, and measurements were recorded throughout the duration of the study. Significant changes in tumor volume or appearance were noted as indicators of treatment efficacy or disease progression.
3.9. Separation of Serum and Measurement of Aspartate Aminotransferase and Alanine Aminotransferase Enzyme Activity
Anesthesia was induced in the mice by intraperitoneal injection of a mixture of xylazine (10 mg/kg) and ketamine (100 mg/kg). After opening the chest with the aid of scissors and sterile forceps, blood was drawn from the heart using an insulin syringe. The collected blood was transferred to a microtube, and the sample was centrifuged for 5 minutes at 15,000 rpm. The activity levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes were measured using a spectrophotometer (BioTek Instruments, Winooski, VT, USA) at a wavelength of 540 nm, with the activity expressed in IU/L units.
3.10. Preparation of Tumor Tissue and Measurement of IL-4, TNF-α, IL-1β, IL-6, and IFN-γ
The tissue sample, weighing 100 mg, was homogenized in 1 mL of a solution formulated with 0.05% Tween 20, 0.05% bovine serum albumin (BSA), 0.0017% phenylmethylsulfonyl fluoride (PMSF), 0.005% benzethonium chloride, 0.0037% ethylenediamine tetraacetic acid (EDTA), and 2 µL of aprotinin per 100 mL. This solution was prepared in PBS at a physiological pH. After centrifuging the homogenate at 10,000 rpm for 10 minutes at 4°C, 50 µL of the resulting supernatant from each tissue sample was collected. The supernatant was then used to measure cytokine concentrations, specifically IL-4, IL-6, TNF-α, IL-1β, and INF-γ. Cytokine levels were determined using an ELISA kit, following the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA) (16).
3.11. Statistical Analysis
The analysis of the obtained values was performed using GraphPad Prism software. The differences between all groups in the study were analyzed using one-way analysis of variance (one-way ANOVA), followed by Tukey's post hoc test for multiple comparisons. Graphs were also generated using the same software. A significance level of 0.05 was applied to determine statistical significance.
4. Results
4.1. Cell Viability Percentage and IC50 Calculation
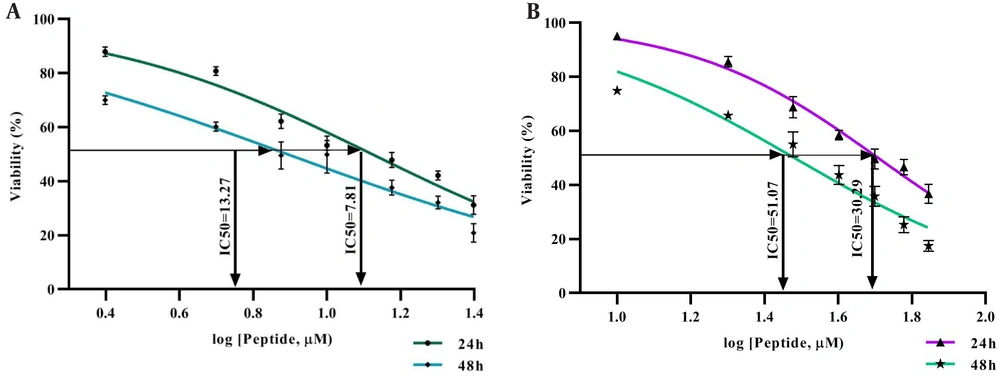
The MTT assay results indicated an inverse correlation between the concentration of the HL-10 peptide and the viability of SiHa cancer cells. Specifically, higher peptide concentrations led to a substantial reduction in cell viability, reflecting an inverse relationship between the peptide dose and the percentage of viable cells. This resulted in a significant decrease in cell viability with increasing concentrations of the HL-10 peptide (P < 0.05). The IC50 value for the HL-10 peptide was 13.27 μM at 24 hours and 7.81 μM at 48 hours, which was lower than the IC50 values observed for carboplatin (Figure 1).
4.2. Expression of Bax, Bcl-2, Cytochrome c, P53, and caspase-3, -9, and 8 Genes
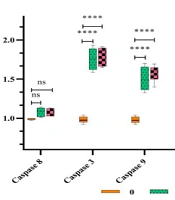
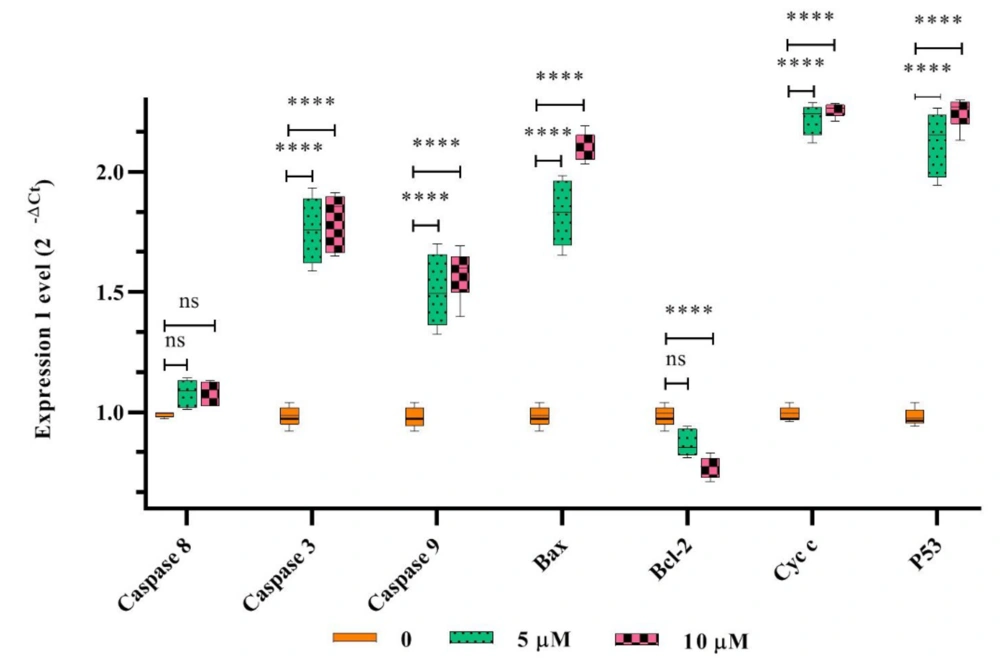
The expression levels of bax, cytochrome c (cyt c), p53, and caspases 3 and 9 genes in SiHa cancer cells increased significantly with higher concentrations of HL-10 peptide (P < 0.05). In contrast, treatment with the HL-10 peptide resulted in a significant reduction in bcl-2 gene expression (P < 0.05). However, there was no significant difference (P > 0.05) in the expression of the caspase-8 gene between the HL-10 peptide-treated cancer cells and the untreated controls (Figure 2).
The expression levels of bax, cyt c, p53, bcl-2, and caspases 3, 8, and 9 genes in SiHa cancer cells exposed to the HL-10 peptide (5 and 10 μM) were compared with those in untreated control cells. Notably, bcl-2 gene expression decreased. Significant differences in gene expression compared to the untreated control are indicated by **** (P < 0.0001). In contrast to the untreated sample, the caspase-8 gene expression did not exhibit a significant elevation with peptide treatment (P > 0.05).
4.3. Tumor Volume Measurement
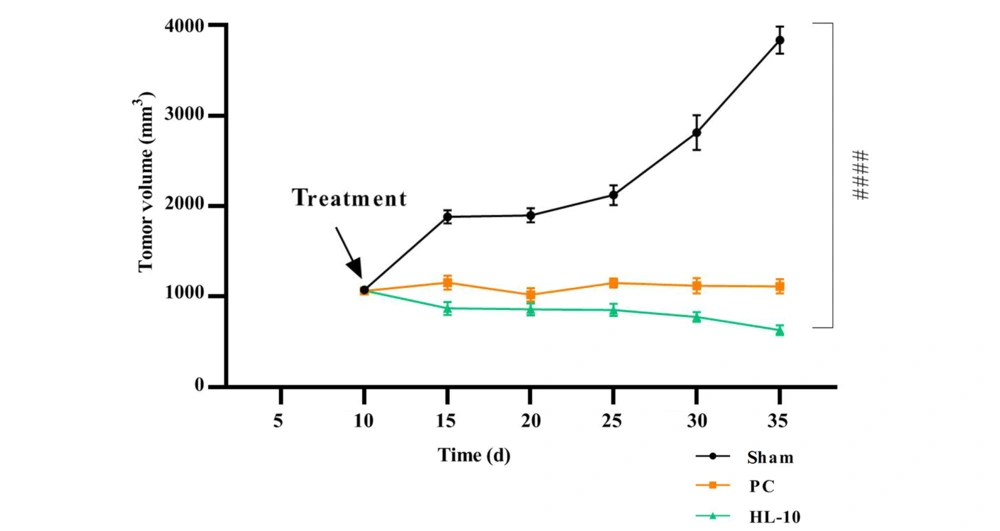
After injecting SiHa cell suspensions into BALB/c mice and allowing 10 days for tumor development, two groups of tumor xenograft model mice were treated: The PC group and the HL-10 peptide group. Tumor volume measurements in the sham group demonstrated a progressive increase over time (Figure 3). Treatment with carboplatin and HL-10 peptide led to a significant reduction in tumor volume compared to the sham group (P < 0.05). However, no significant difference in tumor volume was observed between the HL-10 and PC groups.
Ten days after the induction of the cancer model, the mice were subjected to treatments. The experimental groups included sham (cancer mice without treatment), positive control (PC) (cancer mice treated with carboplatin at 5 mg/kg), and HL-10 (cancer mice treated with HL-10 peptide at 5 mg/kg). The symbol #### denotes a significant difference between the tumor volume values of the HL-10 and sham groups (P-value = 0.0001).
4.4. The Activity of AST and ALT Enzymes
The obtained findings showed that the serum activity of ALT and AST enzymes in the SiHa tumor xenograft mice model treated with carboplatin (PC) increased significantly (P < 0.05) compared to untreated cancer mice (sham). In contrast, the activity levels of ALT and AST enzymes in the HL-10 group did not exhibit a significant increase (P > 0.05) when compared to the Sham group (Figure 4).
Carboplatin and HL-10 peptide treatments influenced the levels of inflammatory markers, including IFN-γ, IL-1β, TNF-α, IL-4, and IL-6 in tumor tissues. The experimental groups were as follows: Sham (mice with cancer tumors without treatment), [positive control (PC): Mice bearing cancerous tissues treated with carboplatin at a dose of 5 mg/kg], and HL-10 (mice bearing cancer cells treated with 5 mg/kg HL-10 peptide). Independent values (ng/g) are presented as means ± standard deviation from six experiments, illustrated in dot plots. **** P < 0.0001.
4.5. Inflammatory Cytokines
According to the obtained results, treatment with carboplatin and the HL-10 peptide in SiHa cancer-bearing mice led to a substantial increase in the levels of IFN-γ, TNF-α, and IL-1β. Conversely, the levels of IL-4 and IL-10 were significantly reduced in cancer mice treated with carboplatin and HL-10 peptide compared to untreated cancer mice (P < 0.05). These findings indicate that carboplatin and HL-10 peptide treatments significantly suppressed the tumor tissue concentrations of IL-4 and IL-10 (Figure 5).
Carboplatin and HL-10 peptide treatments altered the levels of IL-4, IFN-γ, TNF-α, IL-1β, and IL-6 in tumor tissues. The experimental groups included sham (mice with cancer tumors without treatment), [positive control (PC): Mice with cancer tumors treated with 5 mg/kg carboplatin], and HL-10 (mice with cancer tumors treated with 5 mg/kg HL-10 peptide). Independent values (ng/g) are presented as means ± standard deviation from six experiments and are depicted in dot plots. **** P < 0.0001.
5. Discussion
The HL-10 peptide demonstrated a significant cytotoxic effect against SiHa cancer cells compared to the standard drug, carboplatin. Notably, the HL-10 peptide exhibited greater cytotoxicity than carboplatin.
The peptides TsAP-1 and TsAP-2, which lack disulfide bridges and are extracted from the Brazilian scorpion Tityus serrulatus, have been proven to possess both anti-cancer and anti-microbial activities. Synthetic analogs of these peptides exhibit anti-cancer activity against various cell lines, including H157 (oral cancer), H838 (lung cancer), PC3 (prostate cancer), U251-MG (glioma cancer), and MCF-7 (breast cancer) (17).
The mechanisms of action of anti-cancer peptides may include plasma membrane destruction, mitochondrial membrane destruction (18), apoptosis, necrosis (19), indirect immunity (20), involvement of membrane receptors (21, 22), anti-angiogenic effects, and the inhibition of DNA synthesis (23). Wang et al. showed that the HNP-1 peptide (human neutrophil defensins) elicited an immune response against tumors by inducing and utilizing dendritic cells in breast and colon cancer models (24). Additionally, it has been reported that the cell-permeable peptide CR1166, through protein-protein interaction and inhibition of GIPC, decreases proliferation, increases cytotoxicity, and induces apoptosis in pancreatic and breast tumors (22). The paradoxin peptide, extracted from marine fish, promotes apoptosis via caspase-3 activation. Additionally, it disturbs the cell cycle at the G2/M phase, which results in the inhibition of cell proliferation in SCC-4 cells (23). The melittin peptide, extracted from bee venom, induces apoptosis through activating several pathways, including protein kinase Ca2+/calmodulin, transforming growth factor β-activated kinase, and the JNK/P38 MAPK pathway (24).
This study focused on evaluating the expression of several key genes involved in programmed cell death, including bcl-2, bax, p53, cyt c, and caspase-3, -9, and -8, in SiHa cancer cells following treatment with the HL-10 peptide. The expression levels of cyt c, bax, and p53, as well as caspases 3 and 9, increased significantly with higher concentrations of the HL-10 peptide. In contrast, a notable decrease was observed in the expression of the bcl-2 gene.
Moreover, the caspase-8 gene expression remained relatively unchanged in cells exposed to the HL-10 peptide. Kong et al. analyzed the impact of the melittin peptide on the growth rate of SGC-7901 cancer cells using various methods. Their results demonstrated a dose- and time-dependent reduction in cancer cell growth and survival. Additionally, morphological changes in SGC-7901 cells treated with melittin indicated the induction of apoptosis. The activity of caspase-3 in cancer cells treated with melittin was significantly higher than in the control, but the activity of caspase-3 in the non-tumor cell line L-O2 was not affected. The results demonstrated that melittin induces apoptosis in SGC-7901 cancer cells via mitochondrial pathways, aligning with the findings of Kong et al. (25). Furthermore, Tu et al. reported that melittin can induce apoptosis in melanoma cells through the calcium signaling pathway (26). It was also shown that the melittin peptide induces death receptors and inhibits the JAK2/STAT3 pathway in ovarian cancer cells (27).
In the present study, caspase-8 gene expression was not affected, while the growth of SiHa cancer cells was inhibited by the HL-10 peptide. Therefore, the HL-10 peptide stimulated apoptosis through the mitochondrial intrinsic pathway and did not affect the FAS/FASL extrinsic pathway.
After 10 days of injecting SiHa cells into BALB/c mice, two groups of cancer mice (PC and HL-10) were treated. Treatment of cancer mice with carboplatin and HL-10 peptide caused a significant decline in tumor volume in comparison to the group of cancer mice without treatment (P < 0.05). The gonearrestide peptide has been identified as an anti-cancer peptide extracted from scorpion venom. This peptide exhibited no toxicity towards red blood cells and effectively reduced solid tumor volume by arresting the cell cycle in the G1 phase. It also increased the expression of cell cycle regulators, including P27 and P21, in a concentration-dependent manner (28).
Aspartate aminotransferase and ALT enzyme concentrations were measured in serum to evaluate the peptide's influence on liver function in the current research. Our findings indicated a marked elevation in serum ALT and AST levels in the carboplatin group. In contrast, the levels of these enzymes in the HL-10 group of the tumor xenograft model did not exhibit a substantial elevation in comparison to untreated cancer mice. Therefore, our data indicate that treatment with the HL-10 peptide did not result in a significant difference in serum levels of AST and ALT enzymes compared to the control group. Liver toxicity was comparable to that observed in the PC group in the xenograft mice model with SiHa cells, suggesting that HL-10 peptide treatment did not induce additional liver injury.
Rather than directly targeting cancer cells, immunotherapy works by enhancing the host's immune response, thereby facilitating the elimination of malignant cells. This approach is critical in cancer treatment, as it has the potential to prevent tumor progression and metastasis (29). Our results demonstrated that the administration of carboplatin and HL-10 peptide led to the upregulation of IL-1β, TNF-α, and IFN-γ. Studies have demonstrated that TNF-α and IL-1β, while playing crucial roles in the inflammatory process through the recruitment of neutrophils, also have the capacity to induce anti-tumor responses. Research has shown that engineered tumor cells capable of producing TNF-α can inhibit tumor growth in three different mouse models (30).
The present study aligns with research on the modulatory effects of Montivipera bornmuelleri venom on the immune system. In that study, intraperitoneal injection of multiple doses of the venom resulted in measurable changes in the levels of various cytokines, including TNF-α, IFN-γ, IL-1β, IL-10, IL-4, and IL-17, indicating its impact on immune modulation in vivo (31). In another study, exposure to Tityus serrulatus venom resulted in increased levels of IL-6, IL-8, TNF-α, IL-10, and IL-1β in the supernatant of macrophages isolated from mice. This suggests that key toxins in the venom play a significant role in modulating the immune response, particularly within macrophages (11). Several studies have demonstrated the involvement of scorpion venom and its peptide components in the regulation of the immune system (12, 32, 33). Parabutoporin and Opistoporin, two NDBPs present in scorpion venom, have been shown to possess immune-modulating, anti-microbial, and anti-fungal properties, according to the literature. Research has indicated that these peptides can decrease granulocyte superoxide generation, trigger exocytosis, and improve chemotaxis at micromolar levels (33). The findings in this study indicate that HL-10 peptide treatment modulates the immune system by shifting it from a pro-inflammatory Th1/Th17 response towards a more anti-inflammatory Th2/Treg response.
The results obtained from the MTT assay demonstrated that the administration of the HL-10 peptide increased toxicity and decreased cell viability in a dose-dependent fashion. Similar growth inhibitory effects were observed with the HL-7 peptide in MCF-7 and A549 cancer cells, while showing no significant anti-proliferative effect on human peripheral blood mononuclear cells (PBMCs) (15, 28).
![Carboplatin and HL-10 peptide treatments influenced the levels of inflammatory markers, including IFN-γ, IL-1β, TNF-α, IL-4, and IL-6 in tumor tissues. The experimental groups were as follows: Sham (mice with cancer tumors without treatment), [positive control (PC): Mice bearing cancerous tissues treated with carboplatin at a dose of 5 mg/kg], and HL-10 (mice bearing cancer cells treated with 5 mg/kg HL-10 peptide). Independent values (ng/g) are presented as means ± standard deviation from six experiments, illustrated in dot plots. **** P < 0.0001. Carboplatin and HL-10 peptide treatments influenced the levels of inflammatory markers, including IFN-γ, IL-1β, TNF-α, IL-4, and IL-6 in tumor tissues. The experimental groups were as follows: Sham (mice with cancer tumors without treatment), [positive control (PC): Mice bearing cancerous tissues treated with carboplatin at a dose of 5 mg/kg], and HL-10 (mice bearing cancer cells treated with 5 mg/kg HL-10 peptide). Independent values (ng/g) are presented as means ± standard deviation from six experiments, illustrated in dot plots. **** P < 0.0001.](https://services.brieflands.com/cdn/serve/3170b/d39203a26064ca806a2d5b4e42f658d2bedbef09/jjcmb-157039-i004-F4-preview.webp)
![Carboplatin and HL-10 peptide treatments altered the levels of IL-4, IFN-γ, TNF-α, IL-1β, and IL-6 in tumor tissues. The experimental groups included sham (mice with cancer tumors without treatment), [positive control (PC): Mice with cancer tumors treated with 5 mg/kg carboplatin], and HL-10 (mice with cancer tumors treated with 5 mg/kg HL-10 peptide). Independent values (ng/g) are presented as means ± standard deviation from six experiments and are depicted in dot plots. **** P < 0.0001. Carboplatin and HL-10 peptide treatments altered the levels of IL-4, IFN-γ, TNF-α, IL-1β, and IL-6 in tumor tissues. The experimental groups included sham (mice with cancer tumors without treatment), [positive control (PC): Mice with cancer tumors treated with 5 mg/kg carboplatin], and HL-10 (mice with cancer tumors treated with 5 mg/kg HL-10 peptide). Independent values (ng/g) are presented as means ± standard deviation from six experiments and are depicted in dot plots. **** P < 0.0001.](https://services.brieflands.com/cdn/serve/3170b/bffd192533a9e736c404bac2b0f410221ac75d88/jjcmb-157039-i005-F5-preview.webp)