1. Introduction
In the past few decades, diabetes has become the most prevalent and critical metabolic disorder. Its global epidemic has resulted in a significant healthcare burden worldwide. The concern has escalated with the rise in the number of people suffering from diabetes. According to predictions, by 2035, more than 590 million people will be affected by this condition (1). Diabetes has been classified into three main types: Type 1, type 2, and gestational diabetes (diabetes that occurs during pregnancy). Regardless of type, all forms of diabetes result in a prolonged increase in blood sugar levels (2).
It was believed that type 1 diabetes (T1D) occurs due to an autoimmune reaction mediated by T-cells, which attack the insulin-producing β-cells located in the pancreas (3). On the other hand, type 2 diabetes (T2D) is more common among diabetic patients and differs from T1D in its non-autoimmune-mediated mechanism (4). Type 2 diabetes results from a combination of insulin resistance and insufficient pancreatic β-cells to compensate for this insensitivity (5).
One of the strategies of the immune system against viral infection is transient changes in systemic metabolism, which are deficient in individuals with T2D and interfere with the antiviral immune response. The malfunction of systemic metabolism resulting from a viral infection may disrupt glycemic control in T2D (6). Exposure to high glucose levels leads to the impaired phagocytic activity of macrophages and dramatically increases the risk of chronic infection in patients with T2D (7). Viral infection activates the type-I immune response, producing cytokines such as TNF, interferon-gamma (IFN-γ), and IL-6 (6). These cytokines induce transient insulin resistance in muscle and liver. The pancreas compensates for insulin resistance through increased secretion of insulin, which directly promotes the antiviral immune response. In obesity, cytokines can induce insulin resistance. Additionally, several viruses infect the pancreas, negatively impacting insulin production. This may also contribute to the loss of pancreatic β-cell function (6). The association between viral infection and diabetes may reflect an increased risk of pathogenic mechanisms for chronic viruses such as HBV and HIV, leading to insulin resistance linked to chronic inflammation. Some research also indicates that T2D patients are more susceptible to viral infections, as diabetes affects healing. Moreover, hyperglycemia frequently impairs coagulation, fibrin action, body fat, and endothelial function (8).
According to scientific studies, the hypothesis based on the role of viral infections in the occurrence of T1D in genetically predisposed individuals is acceptable. The direct effect of viral infections on immune cells and pancreatic beta cells has been studied, and pathogenic mechanisms have also been suggested.
2. Diabetogenic Viruses
According to research, it has been determined that Coxsackie B, Rubella, Mumps, Epstein-Barr, Varicella-zoster, and Cytomegalovirus (CMV) play a role in the occurrence of T1D (9). Human cytomegalovirus (HCMV) is a member of the Herpes family. Viruses in this family include Epstein-Barr (causing glandular fever) (10), Varicella zoster (causing chickenpox) (11), and Herpes simplex (causing herpes) (12).
The way these viruses spread can be through coughing and contact with blood, urine, feces, or mucous membranes such as those in the mouth and genitals.
3. Viral Mechanisms Trigger or Contribute to Development of Diabetes
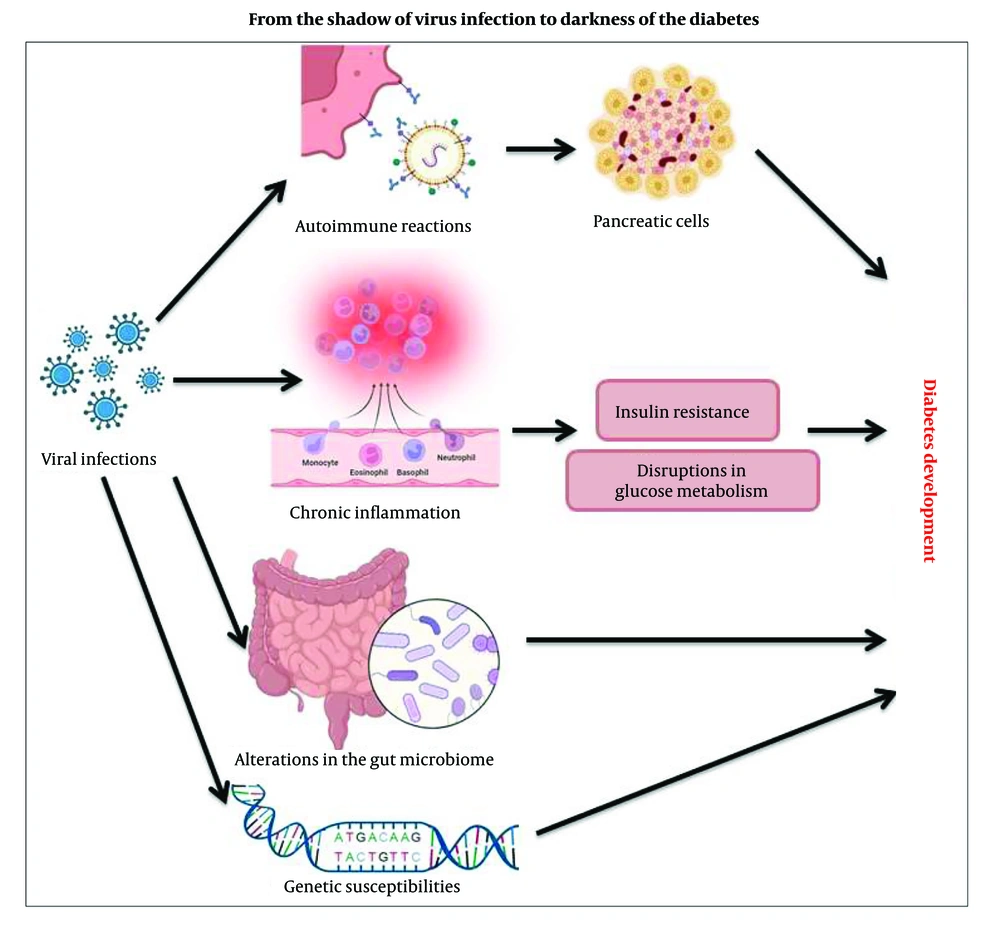
Investigation of viral mechanisms shows that some viruses can trigger diabetes by disrupting glucose metabolism through direct damage to pancreatic beta cells. Viruses can damage pancreatic beta-cells through a process known as cytolysis. When a virus infects a beta-cell, it can trigger the cell to undergo apoptosis (programmed cell death) or lysis (cell rupture) due to the viral replication cycle. This leads to the destruction of the cell and the impairment of insulin production. Additionally, the immune response to the viral infection can further contribute to beta-cell damage, as inflammatory cytokines and immune cells may attack and destroy these cells in an attempt to eliminate the virus (13).
Viruses can influence interactions between host cells and gut microbiota through several mechanisms, including: (1) Interaction of gut epithelial cells with microbiota due to gut tissue tropism and replication within the mucosal lining, contributing to gut dysbiosis (14); (2) increasing or decreasing the abundance and diversity of certain gut microbiota as a result of the host's immune responses, possibly due to the conjunction of viral infection (15); (3) producing antimicrobial peptides or other host factors that shape the microbial community. Viruses, as well as bacteria, archaea, and eukarya, are involved in the gut microbiome and maintain balanced symbiotic or antagonistic relationships through the production of antimicrobials (16); (4) changing microbial metabolism by disrupting nutrient absorption, disturbing energy balance, and causing metabolic disorder. The effects of viral infections may also vary in patients with metabolic problems such as diabetes mellitus. Nutritional status may also impact the pattern of events following viral infection (17); (5) disrupting the integrity of the intestinal barrier, transferring microbes and microbial products from the intestine to the bloodstream, and inducing an inflammatory response that leads to changes in the composition of the intestinal microbiota (18). Figure 1 shows the viral mechanisms that trigger or contribute to the development of diabetes.
4. Autoimmune Reactions Triggered by Viral Infections
Viral infections can cause autoimmune diseases through several mechanisms.
4.1. Epitope Spreading
Since T1D is immune-mediated, it is crucial to identify how the infecting viruses may trigger autoimmunity. Several mechanisms could be at play here. During the early stages of infection, the recognition of pathogen-associated molecular patterns activates innate immunity, leading to the secretion of type-I interferons (IFNs) and pro-inflammatory cytokines. Interferons function in an autocrine and paracrine manner, inducing antiviral responses in infected cells and establishing a virus-resistant state in neighboring cells. The type I IFN response activates the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, leading to the transcription of IFN-stimulated genes (ISGs) that promote the antiviral state. In the later stages of infection, virus replication may cause cell damage, possibly from the release of self-antigens. Antigen-presenting cells take up these self-peptides and deliver them to lymphocytes, which may give rise to autoreactive T-cell clones and autoreactive B-cells producing antibodies.
In brief, viruses infect host cells, present their antigens to T-helper cells (Th cells) via antigen-presenting cells (APCs), and cause the release of cytokines from Th cells. Cytokines affect cytotoxic T-lymphocytes (CTLs) and induce the release of their granzymes to attack infected cells. Then, self-antigens hidden in damaged cells are leaked and presented by APCs to self-reactive T-cells. Finally, self-reactive T-cells attack other non-infected cells carrying these antigens (19).
4.2. Molecular Mimicry
T-cell receptors (TCRs) can recognize and react to both viral antigens and self-antigens that have structural or sequential homology.
4.3. Bystander Activation
Infected cells present viral antigens to virus-specific T-cells. T-cells recognize infected cells and release cytotoxic granules, leading to the cell death of both infected and nearby uninfected cells. The inflammatory milieu further activates bystander cells within the tissue.
5. How About "Viral Survival Tactics: Understanding Mechanisms Used by Viruses to Evade Host Defenses"?
There are several mechanisms by which viruses evade host defenses (20). Table 1 provides examples of viruses along with their corresponding evasion mechanisms.
| Virus | Evasion Mechanism | Ref |
|---|---|---|
| Poxviruses and Herpes viruses | Disrupt presentation of viral antigens by MHC molecules in order to evade control by T-lymphocytes | (21) |
| Measles | Antagonism of the phosphatase PP1 by the measles virus V protein is required for innate immune escape of MDA5 | (22) |
| CVB | Epitope mimicry mechanisms skewing the physiological antiviral response toward autoimmunity | (23) |
| CMVs | Possess a complex DNA structure with a vast genome size (measuring up to 235 kilobases for HCMV). They carry numerous proteins that facilitate their spread and immune evasion. | (3) |
| EBV | EBV stablishes latent infections in B-lymphocytes, making it challenging for the immune system to detect. | (24) |
| Mumps | Molecular mimicry: Identical antigenic epitopes ( nucleocapsid protein) with HLA class II induce cross-reactive antibodies | (25) |
| VZV | The protein product of VZV ORF66 has been found to decrease the expression of MHC class I by interfering with the transport of MHC class I molecules to the cell surface and allow the virus to avoid detection by CTLs and evade immune recognition. | (26) |
Evasion Mechanism of Viruses
6. Viral miRNA Targeting Diabetes Related Gene Expression
MicroRNAs (miRNAs) are non-coding RNAs that are 18 - 22 nucleotides in length and function to regulate gene expression (27) through translational repression or gene silencing (28). Studies have demonstrated that certain viruses are capable of producing viral miRNAs that can regulate the quantities of viral proteins or reduce the expression of cellular factors that possess antiviral properties. There has been limited investigation into the function of viral miRNAs in diabetes. Nevertheless, an investigation published in the Journal of Virology in 2017 indicated that hepatitis C virus (HCV) may be involved in the development of T2D by modulating the expression of several miRNAs in liver cells (29). The study discovered that HCV infection downregulates the expression of miR-29b, a miRNA that is involved in the regulation of glucose metabolism and insulin sensitivity, in human hepatocellular carcinoma cells. The dysregulation of miR-29b expression by HCV may contribute to the development of insulin resistance and T2D in infected individuals (30, 31).
In another study, it was reported that Coxsackievirus B5 disrupts the suppression of pro-inflammatory factors by miRNAs (miR-155-5p and miR-181a-3p produced) in β-cells. This disruption amplifies the antiviral immune response, leading to β-cell destruction and the onset of T1D (32). Furthermore, the study showed that miR-UL112-3p from HCMV targets the insulin-like growth factor 1 receptor (IGF1R) gene, resulting in reduced insulin sensitivity and impaired glucose uptake (28).
7. Viral Life Cycle in Human Cells, Initiate Infection
In this section, the infection mechanism of the HCMV virus is described as an example of the viral life cycle in human cells.
Human cytomegalovirus enters human cells through either direct fusion or the endocytosis pathway. The virus attaches to the cell via interaction (33) between viral glycoproteins and specific surface receptors, followed by the fusion of the envelope with the cellular membrane, releasing nucleocapsids into the cytoplasm (34). These nucleocapsids are then translocated to the nucleus, where viral DNA is released, initiating the expression of IE-1/IE-2 genes (35).
Viral replication and maturation follow, stimulated by and parallel to the accumulation of viral synthesis functions. This process involves encapsulating the replicated viral DNA in capsids, which are then transported from the nucleus to the cytoplasm. Secondary envelopment occurs in the cytoplasm at the endoplasmic reticulum (ER)-Golgi intermediate compartment. This is followed by a complex two-stage final envelopment and egress process that leads to the release of virions by exocytosis at the plasma membrane (36, 37).
8. Silent but Deadly: The Hidden Link Between Latent Viruses and Diabetes
Although previous studies have shown that herpesviruses are stable in the host, they may not be detected in the blood by antibody analysis due to changes in the host's immune system or viral activity (38-41). Scientists have stated that, in most cases, infection caused by the herpesvirus occurs in early childhood (42). An increased antibody response in a person's blood serum does not necessarily mean they are cured, as the virus may be latent and undetectable (43).
By reporting the presence of the CMV genome in lymphocytes and serum islet cell autoantibodies in T1D patients (44), researchers found a strong relationship between CMV infection and diabetes. They also argued that human CMV could play an essential role in the pathogenesis of T1D by examining the cross-reaction of T-cells with beta-cell autoantigen GAD65 (45). In vivo analysis revealed that the virus can infect pancreatic islet cells in both type 1 and type 2 diabetes patients (46).
It is likely that the incidence of active viral infection and T1D in the general population is not very high, which is why many studies on the increased risk of diabetes caused by CMV infection have not received significant attention. On the other hand, due to the observation of CMV infection occurring simultaneously with diabetes in kidney transplant recipients (47), this group of patients has attracted special attention from researchers to investigate the potential relationship between CMV and diabetes.
A defect in antiviral defense causes B-cell dysfunction and increases the risk of developing diabetes after infection with CMV (48).
9. Inflammatory Pathway Triggered by Viral Infection in Diabetic Patients
Several studies suggest that viral infection may play a crucial role in triggering islet-specific autoimmunity and the development of T1D (3). Krogvold et al. reported that live enterovirus antigens have been detected in pancreatic islets of post-mortem T1D cases and live patients (49). Studies have shown that children at genetic risk for T1D have different enterovirus types present in stools before developing islet autoantibodies (50). Mechanisms are predicted to operate during different phases of infection, leading to activation of innate immunity, the transcription of ISGs, and the production of cytokines. Marroqui et al. showed that type I interferons are key players in pancreatic β-cell dysfunction in T1D (51).
Enterovirus infection in T1D patients impacts pancreatic islets and has a potential role in causing islet-specific autoimmunity. Viral antigens have been found in pancreatic islets from both deceased and living T1D patients, and at the onset of clinical symptoms, ISGs and human leukocyte antigen class-I antigens are hyper-expressed in the islets, with memory CD8 T-lymphocytes among infiltrating cells. Taken together, researchers suggest that enterovirus infection may be a pre-requisite for T1D development (52). Within islets, macrophages and dendritic cells are involved in an inflammatory process in which numerous immune system components play a part (insulitis).
In the case of T2D, persistent CMV infection may play a role in the pathogenesis of T2D. Cytomegalovirus induces the accumulation of differentiated CD4+ and CD8+ T-cells to produce pro-inflammatory cytokines, resulting in a more pro-inflammatory environment and ultimately, accelerated immunity (53). Specific pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) can harm pancreatic β-cells (54, 55). It may cause inflammation that contributes to insulin resistance by promoting lipolysis, disrupting insulin signaling, and decreasing the expression of GLUT4. When there is an infection, macrophages release TNFα to signal other immune cells and activate an inflammatory response (56-58).
10. The Widespread Reach of Human Cytomegalovirus: How Virus Spreads from Local Sites to Entire Body
The HCMV-infected epithelial cells at the initial infection site likely rapidly produce the virus, which then spreads to adjacent cells at these local sites. Monocytes infected with HCMV, either as they are patrolling or perhaps by a free virus, infiltrate various organ tissues and spread throughout the body. These monocytes differentiate into macrophages, allowing for organ persistence, infection of new epithelial cells, and virus release in various body fluids. Some of these infected monocytes infiltrate the bone marrow, where they enable the establishment of latency in CD34+ HPCs. During a reactivation event, monocytes develop from CD34+ HPCs, spreading the virus throughout the body (59).
11. Conclusions
In conclusion, this investigation highlights the complex relationship between viral infections and diabetes, revealing how viruses such as Coxsackie B, Rubella, Mumps, Epstein-Barr, Varicella-Zoster, Cytomegalovirus, and Herpes Simplex contribute to the pathogenesis of diabetes. These viruses employ various evasion mechanisms, including molecular mimicry, immune evasion proteins, and the establishment of latency, which disrupt the host’s immune responses and metabolic processes. Viral infections can lead to insulin resistance and alter systemic metabolism, particularly in T2D, by impairing pancreatic function and affecting gut microbiota. Furthermore, chronic viral infections may exacerbate hyperglycemia, increasing the risk of diabetes-related complications. Understanding these intricate interactions is essential for developing targeted antiviral strategies and vaccines that could mitigate the impact of viral infections on diabetes management. This underscores the need for ongoing research to unravel these relationships and improve therapeutic approaches against these dynamic pathogens.