1. Background
Environmental sonochemistry is an example of advanced oxidation processes (AOPs) and deals with the removal of toxic and hazardous organic compounds from contaminated waters (1, 2). Textile industry wastewaters have considerable amounts of organic dyes, which are resistant to conventional biological treatments. Furthermore, other physical and chemical processes, such as adsorption and coagulation, merely transfer contaminants to a secondary phase that requires more treatment. The advantages of using ultrasound are based on simplicity of its use, producing no secondary waste and good penetration of ultrasonic waves in non-transparent solutions (3-8).
Malachite green (MG) is used as an agent for food coloring, an additive to food, an anthelminthic, medical disinfectant and also as a dye in the silk, leather, cotton, paper, acrylic and textile industries. However, MG has become a highly controversial compound recently, owing to the risks it creates to those who consume treated fish, including its influences on the reproductive and immune system and its carcinogenic and genotoxic properties. The use of MG has been forbidden in several countries; for instance, it is not approved by the U.S. Food and Drug Administration. However, it is still being used in the world because of its low cost, availability and efficacy (9-11).
Cavitation is the origin of sonochemical effects, creating micro bubbles. After ultrasonic waves enter in to a liquid, the cavitation phenomenon results in formation, growth, and eventually collapse of micro bubbles generating highly localized temperatures and pressures (hot spot approach). Hence, intense energy is available for chemical and mechanical effects in the solution.
The generated reactive oxygen species from the dissociation of water molecules under ultrasonic irradiation, especially hydroxyl radicals, have the main role of degradation and mineralization of various organic contaminants such as the dyes. In the hot spot model, three regions exist including the gaseous nuclei, the interfacial regions and the bulk solution, which are dependent on the pollutant type. The degradation can occur mainly in a particular region (12-15).
Various models have been developed for description of the sonochemical process mechanism, based on the pseudo-first order kinetics assumption. Moreover, development of a kinetics model for a process based on intrinsic elementary reactions is one of the best methods, due to the exact understanding of the reactions in the process and the effects of operational parameters. However, sometimes this kind of modeling is difficult owing to the diversity of reactions, especially for a complicated process. Thus, an empirical kinetics modeling of the process via non-linear regression analysis can be applied in such cases. The validity of this empirical model was evaluated by comparison of experimentally and theoretically calculated data (16-20).
2. Objectives
The objective of the present study was to develop an empirical kinetics model to predict apseudo-first order rate constant (kap) of the MG sono-degradation as a function of initial concentration of dye [MG0], temperature (T), power density (P/V) and mechanical agitation (MA) using non-linear regression analysis. Finally, this model can be used in order to determine the required electrical energy (EEO) for the process at different operational conditions.
3. Materials and Methods
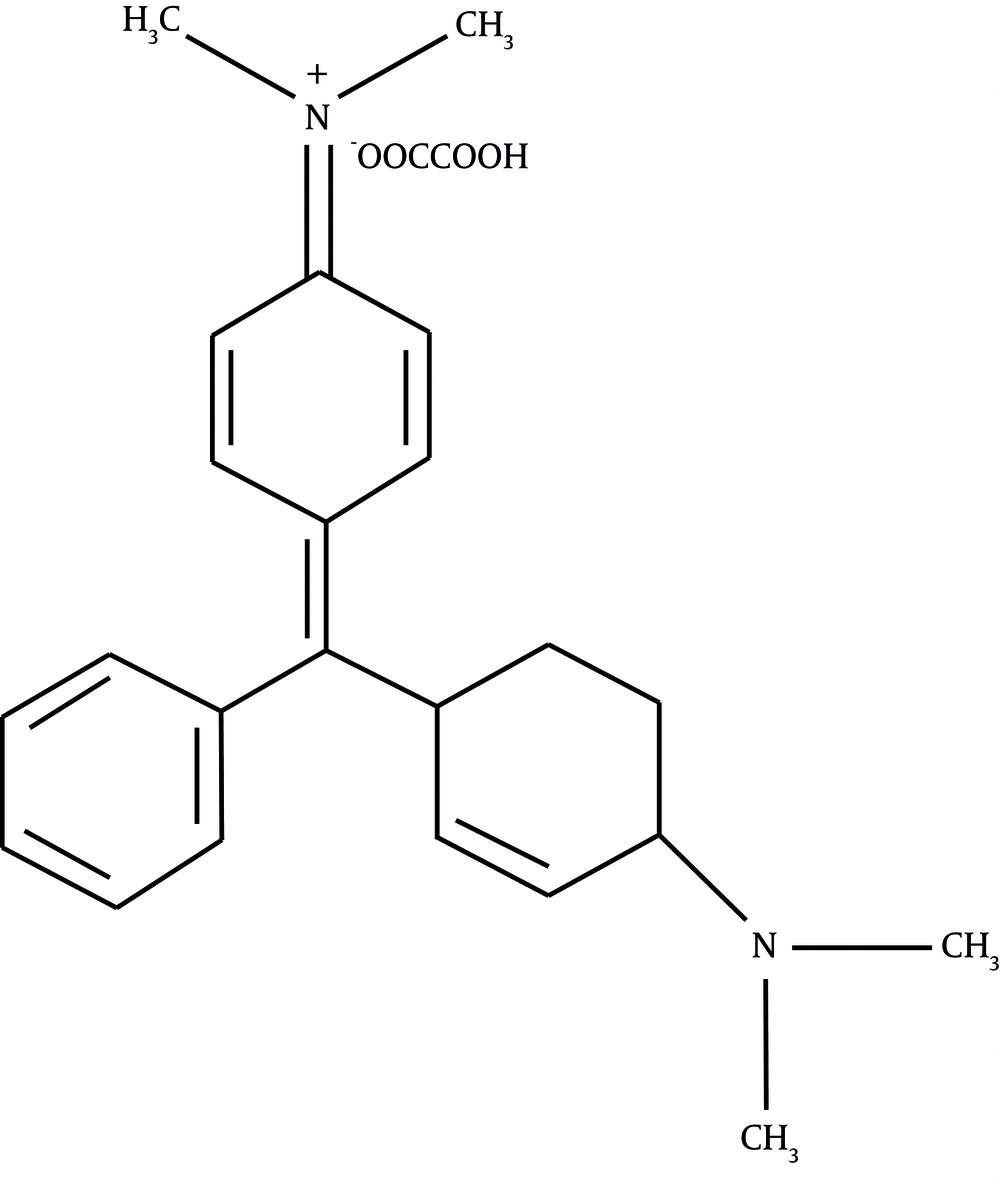
Malachite green oxalate (C.I. 42000), whose structural formula is shown in Figure 1, was obtained from Panreac Co. (Spain) and used without further purification. All solutions were prepared with double-distilled water by dissolving the required amount of MG. The ultrasonic bath was purchased from Elma (GmbH) with an operating frequency of 35 kHz and with a rated output power of 170 W. The efficiency of the bath was determined by the calorimetric method as 28.8%, which indicates that the actual power (P) dissipated in the bulk solution is 49 W (13). Power density was calculated by dividing the actual power dissipated in the bulk solution to the volume of the dye solution. Mechanical agitation was used during the experiments. The operational conditions of the experiments have been summarized in Table 1. In each experiment, a specific volume of the MG solution, with a specific concentration, was poured into the ultrasonic bath. A mechanical stirrer was used in the solution, and the sonication was started. At distinct reaction intervals, 5 mL of the sample was withdrawn and the concentration of MG was determined by the spectrophotometry method using a spectrophotometer (Ultrospec 2000, Biotech Pharmacia, England) at 617 nm.
| Operational Parameters | Values |
|---|---|
| MG initial concentration, m gL-1 | 4.89 - 9.87 |
| Temperature, K | 294 - 307 |
| Power density, W mL-1 | 0.049 - 0.163 |
| Mechanical agitation, rpm | 75 - 400 |
Operating Conditions of Experiments
4. Results
The apparent rate constants (kap) of MG degradation were estimated using the pseudo-first order kinetics assumption from the slope of ln ([MG]o/[MG]) versus irradiation time at different operational conditions (13). In all cases, the R2 (correlation coefficient) values were higher than 0.99, which confirmed the proposed pseudo-first order kinetics for the process.
The relationship between kap and the initial concentration of MG, power density and mechanical agitation can be modeled with the power law-type of empirical Equations, which have the general form of:

Using nonlinear regression analysis with Polymath 5.1, model parameters were calculated and reported, and are shown in Table 2. In addition, the dependence of kap on temperature can be calculated from the Arrhenius Equation and activation energy (Ea) was 30.95 kJmol-1 (13). Therefore, kap can be modeled as the following Equation:

| Operational Parameters | A | Q | R2 |
|---|---|---|---|
| MG Initial concentration, m gL-1 | 0.0463 | 0.948 | 0.999 |
| Power density, W mL-1 | 0.0505 | 0.547 | 0.983 |
| Mechanical agitation, rpm | 0.0022 | 0.307 | 0.999 |
Model Parameters for Predicting kap
With known values of [MG]0 (m gL-1), (P/V) (W mL-1), (MA) (rpm) and T (K), we can calculate k0 as 15.75 × 103. Substituting these values into Equation 2, we obtained:

The results of this work are similar to the information found in the literature. Kim et al. (4) have reported the value of m as -1.87 for sonochemical degradation of dibenzothiophene in the initial concentration range of 3.3 × 10-6 to 7.7 × 10-6 M. Furthermore, Sivakumar and Pandit (7) found the value of power density to be 0.6418 for the degradation percent of Rhodamine B with four different ultrasonic systems. Also, for sonochemical decomposition of dibenzothiophene in aqueous solution, the activation energy was 12.6 kJ mol-1, in the temperature range of 288 - 323 K (4).
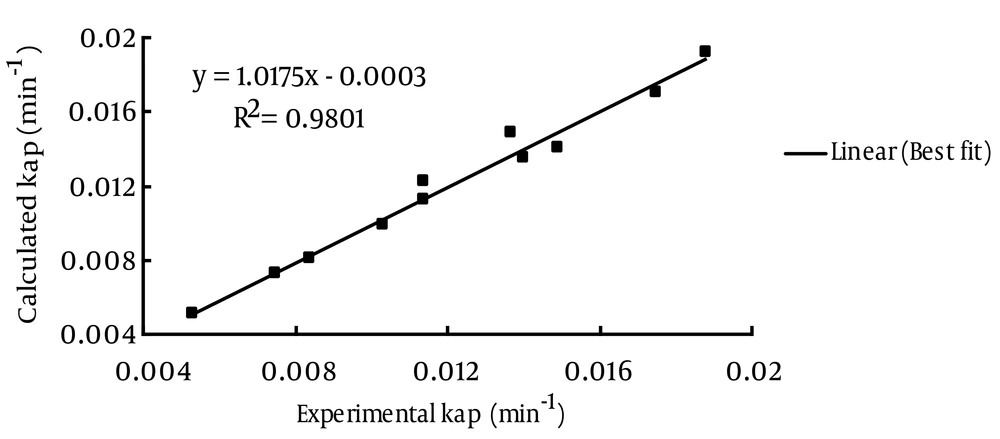
For evaluation of Equation (3) in predicting kap in different conditions, a comparison between experimental and calculated kap for sonochemical degradation of MG is shown in Figure 2.
For experimental details refer to Table 3.
| Operational Parameters Variation | EEO (Experimental) | EEO (Calculated) |
|---|---|---|
| MG initial concentration (m gL-1)a | ||
| 4.89 | 633.79 | 658.21 |
| 6.82 | 870.40 | 902.30 |
| 9.87 | 1231.70 | 1280.66 |
| Temperatureb, K | ||
| 297 | 572.63 | 581.97 |
| 302 | 438.12 | 463.84 |
| 307 | 373.03 | 383.18 |
| Power densityc, W mL-1 | ||
| 0.07 | 818.05 | 764.32 |
| 0.098 | 952.99 | 879.31 |
| 0.163 | 1157.45 | 1131.89 |
| Mechanical agitationd, rpm | ||
| 75 | 777.14 | 812.34 |
| 400 | 466.28 | 485.29 |
Experimental and Calculated Amounts for EEO
The EEO can be calculated from the following Equation for a batch-type reactor:

Where Pel is the input power (kW) to the AOPs system, t is the irradiation time (min), V is the volume of water (L) in the reactor and C0 and C are the initial and final pollutant concentrations, respectively (21). Assuming first-order kinetics:

A comparison between predicted EEo values estimated from kinetics modeling (Equation 3) and EEo values obtained from experimental data is shown in Table 3.
5. Discussion
MG is not a volatile compound; thus, its presence in the gas phase of a cavity is unlikely. Consequently, the major route for its degradation is oxidation by hydroxyl radicals in the interfacial area. Moreover, the applied frequency of sonication is low; hence, the collapse of bubbles is more violent. However, the delayed growth and longer collapse duration permit radical scavenging and recombination reactions at the interfacial sheath, hence, inhibiting reactive species into the bulk solution. As a result, the dye degradation is increased by adsorption on the bubble-water interface (13, 22). Then, MG, a cationic dye in the basic class, can be degraded by ultrasonic irradiation, and kap can be predicted at different operational parameters, utilizing nonlinear regression analysis to obtain the empirical kinetics model. For cost evaluation, electrical energy per order (EEO) based on experimental and calculated data was obtained. The results of this study (Table 3) show that the EEO is directly proportional to the MG concentration and power density, and inversely proportional to temperature and mechanical agitation. From Figure 2, it can be seen that the results obtained from the model (Equation 3) are in good agreement with the experimental data.
As mentioned previously, the main route for MG degradation is the oxidation by hydroxyl radicals in the interfacial area; the MG degradation is limited by the available interfacial area generated at the constant sonication condition (5, 22). An explanation of the effect of power density on EEO seems to be necessary. Although kap increases with enhancement of power density, the solution volume becomes lower in the high power densities than in the low power densities. The effect of decreasing of volume is predominantly seen in Equation 5, and the EEO becomes low with enhancement of power density. The increase in temperature will raise the vapor pressure and so cause easier cavitation but less violent collapse; in this temperature range, the influence of easier cavitation is predominant. The influence of sonication on sono-degradation of MG was enhanced by mechanical stirring, owing to the dispersion of the cavitation bubbles and easier diffusion of the dye to the interfacial area (13).
Goel et al. (23) have reported EEO values for ultrasonic degradation of trichloroethylene, toluene, styrene and eosin B in aqueous solution with an initial concentration of 50 mgL-1 in an ultrasonic bath (100 W) as 4,382; 6,643; 7,538 and 7,532 kWh per m3 per order, respectively. In our work, EEO values for ultrasonic degradation of MG with an initial concentration of 5 mg L-1 in an ultrasonic bath (170 W) at different operational parameters changed from 373 to 1,232 kWh per m3 per order. The low EEO and the difference between our data and the other studies could be related to the difference in the experimental set-up of the ultrasonic bath, such as the mechanical stirrer, direct dissipation of ultrasonic waves to the solution and also various operational conditions.