1. Background
Textile industrial wastewater contains various pollutants such as organic matters, dyes and nutrients. These pollutants are produced in different steps such as washing, sizing, belching, dyeing, printing, and other operations. The most important problem in relation to textile industrial wastewater treatment is residual dye removal from none-permanent dyes. During the dyeing processes, 5% - 20% of dye enters wastewater due to lack of stability (1-4). On the other hand, these dyes may cause adverse effects such as aesthetic, carcinogenic and toxicity and consequently can be harmful to human beings, animals and plants (5-7).
Nowadays, about 26,000 types of dyes are produced worldwide (8). The major environmental concern for industrial wastewater is related to very intense dye content as well as a high chemical oxygen demand (COD) rate (9). Therefore, continuous and constant discharging of wastewaters into water resources can cause eutrophication and aquatic life disturbance (10). Azo-dyes have different forms such as direct, acid, base, reactive, disperse, metal-complexed, mordant and sulfur dyes (11). Azo dyes, which contain one or more azo bonds (N = N), are among the most widely used synthetic dyes and usually become major pollutants in textile wastewaters (12).
Common methods of physical and chemical treatment such as chemical precipitation, coagulation and adsorption by activated carbon cannot destroy this substances and only can transfer this compound from one phase to another (11, 13, 14). Also, common biological systems cannot treat wastewaters because most of the resistant pollutants in textile industrial wastewater are toxic for living organism and have growth inhibitory properties (15, 16). In recent years, advanced oxidation processes have developed significantly. Generally, advanced oxidation processes include all processes in which active hydroxyl radicals (OH°) are produced using different methods. Hydroxyl radicals that have very high oxidation power can remove most of the pollutants, especially organic pollutants thoroughly (17). The photo Fenton process includes activated hydrogen peroxide with iron salt and removes pollutant and transfers them to CO2 and H2O and other harmless substances by producing hydroxyl radicals (18). The photo-Fenton process was conducted using H2O2, Fe (II), and UV radiation (580 nm) (19). In this process, OH° was produced in the presence of ferrous Fe (II) and peroxide hydrogen as reductive and oxidative reagents, respectively. An increase in the degradation rate of the pollutant can be attributed to the oxidation of Fe (III) to Fe (II) by UV radiation, producing OH° according to the following mechanism (Equation 1) (20):
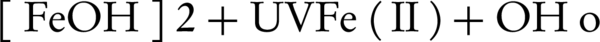
High efficiency rates of these methods are considered by scientists and researchers of water and industrial wastewaters treatment. Other studies show that the photo Fenton process has a higher efficiency when compared to the UV/H2O2 process or photocatalytic UV/H2O2 process (17, 21). Short reaction time, low chemical consumption, and removal capability of resistant pollutants such as polyaromatic organic compounds are the other advantages of this process (15, 22).
In recent years, the photo Fenton process has been used as an environmental advocating technology and cleaning process to treat different kinds of wastewater (23, 24). The study of Perez et al. indicated that combination of Fenton and photo Fenton reactions has a high efficiency in textile industrials wastewater treatment (25). The study of Zheng et al. in investigating oxidation acidic dye Eosin Y by the photo Fenton process showed that at pH = 3.5 and reaction time of 90 minutes, 94.1% of dye was removed and the degradation rate was dependent on dye concentration (26). The study of Huang et al. on comparative survey of oxidation of dye-reactive black B by different advanced oxidation processes including Fenton, electro-Fenton and photo-Fenton indicated that Fenton alone made 70% of mineralization, whereas photo Fenton made 93% mineralization with rapid and more complete degradation of dye solution (27). The study of Mahavi et al. in investigating removal of reactive red 120 and direct red 81 (DR 81) dyes from aqueous solutions by pumice showed that pumice can be used for sorption of both RR120 and DR81 dyes from aqueous solutions (6). The study of Ali et al. in investigating removal of DR 81 dye from aqueous solution by native and citric acid modified bamboo sawdust - (a kinetic study) and equilibrium isotherm analyses showed that the developed adsorption system is inexpensive, ecofriendly and efficient for the removal of dye from any water due to its small adsorption time, good capacity of adsorption of adsorbent and working at 7 pH; a natural pH of most of natural water resources (28). Also, the study of Heravi et al. investigated equilibrium and kinetic studies of DR 81 biosorption onto modified silk maze as an economical biosorbent indicated that the modified silk maze had the maximum monolayer adsorption capacity, 5.5 mg/g (29).
However, no studies until today have been conducted on removing DR 81 dye from aqueous solutions using the photo- Fenton process. The Fenton and photo-Fenton processes have significant importance because of their high speed in removing contaminant materials and can be used for the treatment of wastewater, especially textile and dyeing wastewater (30). The study of advanced oxidation processes on removing diverse and new chemical structures requires comprehensive information about the industrial application of this process.
2. Objectives
The aim of this study was to investigate the removal efficiency of DR 81 dye using the Photo- Fenton method and also determine the optimal process conditions based on removal efficiency and the impact of various factors such as pH, concentration of ferrous sulfate heptahydrate, hydrogen peroxide concentration, initial dye concentration, and reaction time.
3. Methods
3.1. Materials
Direct red 81 dye with chemical formulation of C29H19N5Na2O8S2 molecular weight of 675.60 g/mol, and chemical materials including hydrogen peroxide (30%), ferrous sulfate heptahydrate (FeSO4.7H2O), sulfuric acid (98%), and sodium hydroxide with a laboratory purity grade were purchased from the Merck Company in Germany.
3.2. Procedures
This was a cross-sectional study performed in a laboratory scale to investigate the impact of the photo-Fenton process by determining the effects of other variables on DR 81 dye removal. The experiments were designed on the basis of one factor at the time to obtain optimum parameters. The number of samples were 25 with pH in 4 levels (3, 5, 7, 11), Fe (II) in 6 levels (0, 10, 30, 50, 100, 150 mg/L), hydrogen peroxide in 5 levels (0, 20, 50, 100, 150 mg/L), dye concentration in 4 levels (50, 100, 150, 200 mg/L), and reaction time (15, 30, 45, 60, 90, 120 minutes). Each sample was repeated 3 times to improve the accuracy and precision of the experiment. Therefore, the total number of samples was 75, and the results were presented based on the average of total number of samples. The reactor used in the experiments was a glass reactor with the volume of 1 Lit equipped with a magnetic stirrer to uniform the solution. A UV lamp with intensity of 8 W/m2 was used in the experiments Figure 1. A dye removal rate was measured using the spectrophotometer DR5000 model at the wavelength of 520. HCl and NaOH used for pH adjustment, and pH meter hatch were used for pH measurement. Dye removal efficiency was calculated by the following formula:
Equation 2.
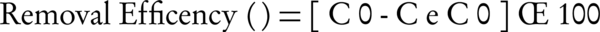
C0 = Primary absorption percentage (mg /L).
Ce = Final absorption percentage (mg /L).
Finally, the data were analyzed using the Excel software to determine the effects of different variables on the efficiency of the decolorization process.
4. Results
4.1. The Effect of pH on the Removal Efficiency of Direct Red 81 Dye Using the Photo-Fenton Process
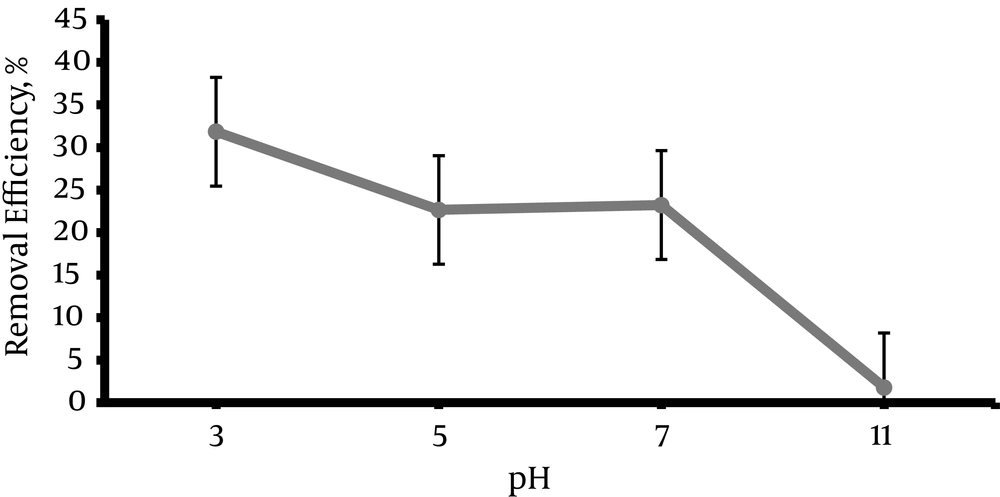
The experiments were performed at 4 levels of pH (3, 5, 7, 11) in order to determine the impact of pH on the DR 81 dye removal rate. As shown in the Figure 1, the maximum removal efficiency (31.85%) was at pH = 3. The removal efficiency decreased by increasing the pH to 5 after which pH was set on neutral value. After that, increasing pH and the alkali environment (according to Figure 2) resulted in decreasing the removal efficiency rapidly down to 1.78%
4.2. The Effect of Fe (II) Concentration on the Removal Efficiency of Direct Red 81 Dye Using the Photo- Fenton Process
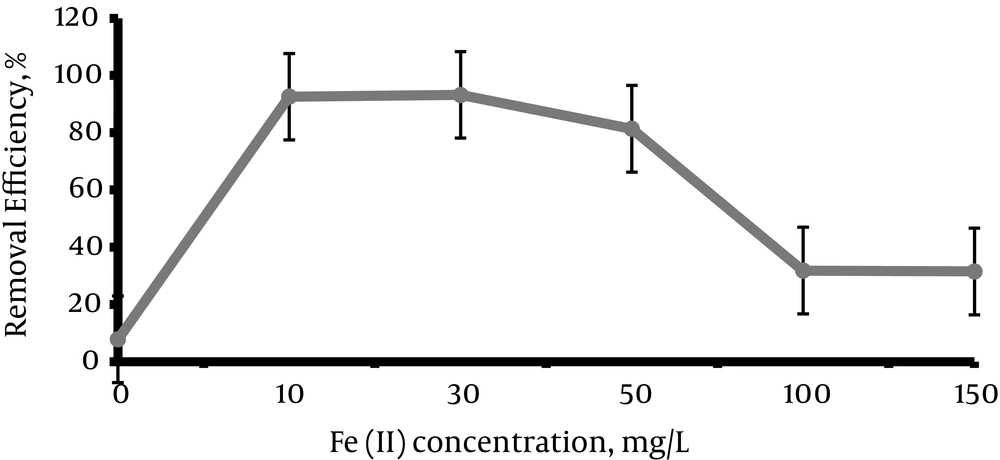
The concentration of Fe (II) was investigated at different levels (0, 10, 30, 50,100, 150 mg/L). According to Figure 3, maximum removal efficiency occurred at the concentrations of 10 mg/L and 30 mg/L. According to the results, removal efficiency was increased by increasing the concentration up to 30 mg/L. Then the efficiency was reduced and in concentrations of 100 to 150 mg/L remained at a fixed value.
4.3. The Effect of H2O2 Concentration on the Removal Efficiency of Direct Red 81 Dye Using the Photo-Fenton Process
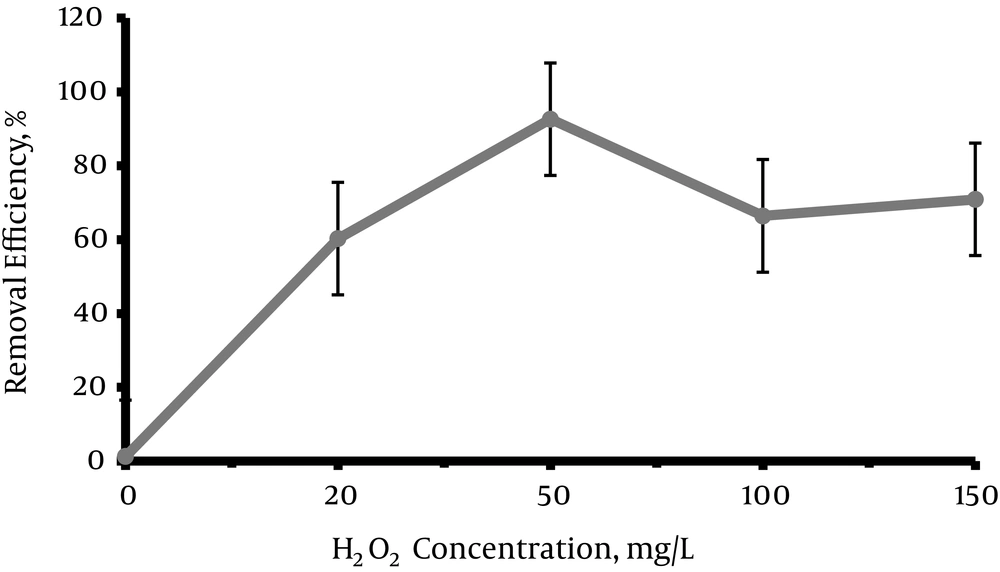
The concentrations of H2O2 in the experiment were 0, 20, 50, 100, 150 mg/L. According to the Figure 4, dye removal efficiency increased by increasing the concentration of H2O2 from 0 to 50 mg/L and reached to 92.59% and then the efficiency decreased and reached to 70.89% at the concentration of 150 mg/L.
4.4. The Effect of Initial Dye Concentration on the Removal Efficiency of Direct Red 81 Dye Using the Photo Fenton Process
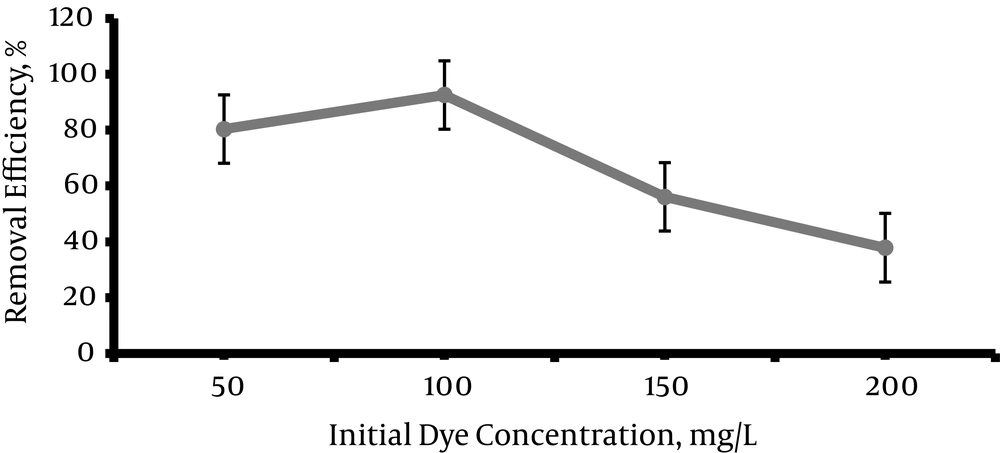
Four levels of dye concentrations (50, 100, 150, 200 mg/L) were experimented in this step. According to Figure 5, maximum removal efficiency was at the concentration of 100 mg/L and reached 92.59%. Then removal efficiency was reduced by increasing the concentration and reached to 37.87% at concentration of 200 mg/L.
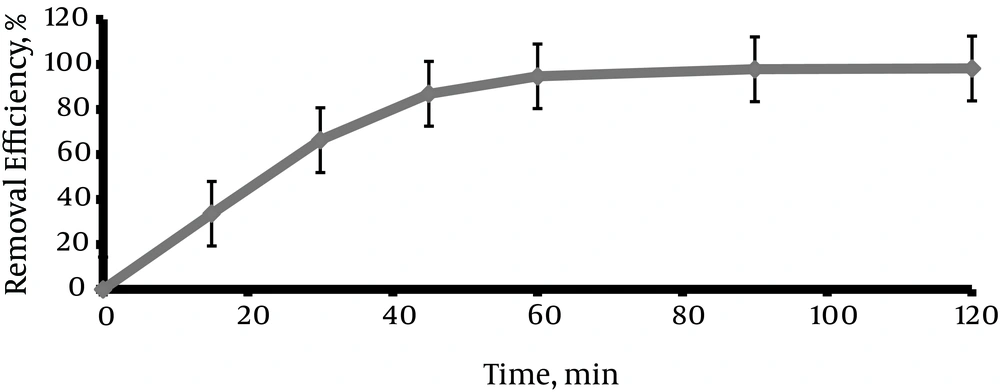
4.5. The Effect of Reaction Time on the Removal Efficiency of Direct Red 81 Dye Using the Photo Fenton Process
The experiments were performed with different reaction times of 0, 15, 30, 45, 60, 90, and 120 min. As shown in Figure 6, removal efficiency increased by increasing reaction time, then the decolorization rate increased at the beginning and declined in the final period.
5. Discussion
According to previous studies on advanced oxidation processes, photochemical and photocatalytic, pH and contact time are considered as effective parameters on these processes (23, 31).
5.1. The Effect of pH on the Removal Efficiency of Direct Red 81 Dye Using the Photo Fenton Process
The value of pH is one of the most important environmental factors that influences the chemical process (31-33). According to Figure 2, maximum removal efficiency was in pH = 3. The removal efficiency was reduced rapidly by increasing pH in an alkali environment because of pH is a effective factor on the hydroxyl radical formation and Fe (II) concentration. Fast decreasing of efficiency in alkaline pH can be explained by the formation and precipitation of Fe (OH)3 that inhibit the development of the photo-Fenton reaction (25). The study of Zheng et al. (26), Peternel et al. (34), and Kang et al. (35) also showed that the maximum removal efficiency in the photo Fenton process occurred in the acidic pH about 3, because the maximum hydroxyl radical formation occurs in this pH and it is also more stable in this condition, This result confirms the results of the present study. At a pH lower than 3, the generated ferric hydroxide reacted with H2O2, which resulted in a decrease in the OH°, eventually decreasing the removal efficiency (Equation 3) (36).
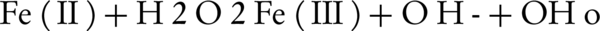
5.2. The Effect of Fe (II) Concentration on the Removal Efficiency of Direct Red 81 Dye Using the Photo Fenton Process
According to Figure 3, the removal efficiency increased by increasing the concentration of Fe (II) to 10 mg/L, and reached to 92.6%. After that, the removal efficiency was almost constant up to Fe (II) = 30 mg/L. Then, the removal efficiency was reduced by increasing the concentration of Fe (II). These results agreed with the study results of Zheng et al., (26) and Elmorsi et al. (37). The Fe (II) additive effect is due to the catalytic properties of Fe (II) and more production of hydroxyl radical by increasing the concentration of Fe (II) (27, 38). However, the addition of extra Fe (II) concentrations more than 10 mg/L resulted in a reduction in the process efficiency and acted as a scavenger for the OH° (39).
5.3. The Effect of H2O2 Concentration on the Removal Efficiency of Direct Red 81dye Using the Photo Fenton Process
According to the Figure 4, removal efficiency increased and reached 92.59% by increasing the concentration of H2O2 to 50 mg/L. Then removal efficiency was reduced by increasing the concentration, and reached 70.89% in the concentration of 150 mg/L. In the numbers of studies, the concentration of H2O2 has been a very important factor for the oxidation reaction of organic compounds and with increasing the concentration of H2O2 to the environment optical dispersion of organic material increased, however up to a certain point. The reason is that the H2O2 concentration more than the optimal concentration may enhance the production of OH-, which consumes the OH° and decreases the removal rate (19, 37, 38). Rahmani et al. (40) revealed that BV16 and RR120 removal rates were accelerated by increasing the concentration of H2O2 until to the optimum level, after which point the dye removal efficiency decreased. Many studies including the study of Daneshvar et al. (3), Zheng et al. (26), and Li et al. (41) also confirmed the results of the present study.
5.4. The Effect of Initial Dye Concentration on the Removal Efficiency of Direct Red 81 Dye Using the Photo Fenton Process
According to the Figure 5, removal efficiency increased (92.6%) by increasing the initial dye concentration up to 100 mg/L, but, after that, decolorizing efficiency decreased with increasing an initial dye concentration and reached 37.9% in the concentration of 200 mg/L. Many studies have shown that decolorizing percent could be reduced by increasing the initial dye concentration. The reason for this reduction is decreasing in penetrating of photons in dye solution due to increase in UV absorption by dye solution. These results corresponded with the results of this study (26, 41-43).
5.5. The Effect of Reaction Time on the Removal Efficiency of Direct Red 81 Dye Using the Photo Fenton Process
The reaction time is one of the most effective factors in the advanced oxidation processes. Figure 6 shows the effect of reaction time in determining optimal conditions of experiment. According to the illustration, dye removal rate is very low (33.65%) in the first 15 minutes, but increases significantly as time goes by and results in further formation of hydroxyl radicals, and maximum efficiency (98.2%) is observed in 120 minutes that corresponds with the other studies (27, 44, 45). The decolorization rate in this study, as in many other studies, increases by increasing the reaction time, and maximum removal efficiency occurs in the initial times, however, the efficiency does not increase significantly in final phases (35, 38, 44). According to time and economical aspects, removal efficiency does not increase significantly in the final phases, and only the initial 45 minutes is the optimum time.
5.6. Conclusions
The results show that removal efficiency by the photo Fenton process increased by increasing the Fe (II), H2O2, and initial dye concentration to a certain limit, due to further formation of hydroxyl radical. The removal efficiency was also increased by increasing the reaction time. In this study, pH = 3, Fe (II) concentration of 10 mg/L, H2O2 concentration of 50 mg/L, dye concentration of 100 mg/L, and reaction time of 45 minutes were considered as the optimal parameters. Moreover, the dye removal efficiency from aqueous solutions was more than 85% at optimal conditions. Therefore, the study results indicated that the photo Fenton process could be used as an effective method to remove DR 81 dye from industrial wastewater. Due to the financial limitations of the study, we only determined the removal efficiency of DR 81 dye using a spectrophotometer (DR 5000). Therefore, it is highly recommended that future studies use the gas chromatography-mass spectrometry (GC-MS) method to determine the intermediate compound, which may be produced from the photo-Fenton reaction.