1. Background
Escherichia coli (E. coli) are facultative anaerobic bacteria, which are Gram-negative, rod-shaped, and motile by peritrichous flagella. Common classification of E. coli species is based on the mechanisms of pathogenicity, virulence factors, O and H antigenic serotyping, and clinical syndromes (1). E. coli may be expressed in different virulence factors related to different pathotypes, isolated from bovine infections, such as diarrhea and septicemia in calves and urinary infection, metritis, and mastitis in cows.
Microbial cell colonization of biofilms occurs on surfaces, and gentle rinsing cannot remove them. The similar structure of biofilms to the polysaccharide matrix material can support the survival and growth of bacteria in sessile environments (2). Pathogenic bacterial biofilms on indwelling devices or tissues cause infections and increase antimicrobial resistance and host immune responses (3). Production of these bacteria on medically relevant surfaces may be difficult to eradicate, resulting in the stability of infection (4).
Previous studies show that subinhibitory concentrations of antibiotics can trigger the formation of biofilms (5, 6). Pathogenic bacteria are embedded in a self-produced extracellular protein matrix and some micromolecules, including exopolysaccharide (EPS) and DNA (7, 8). Various definitions of biofilm indicate three major constituents, including the surface, microbes, and slime EPS; biofilm production can be terminated by removing any of these constituents (9).
Several superficial proteins in different organisms contribute to early biofilm formation and attachment to eukaryotic cell hosts. These surface determinants contribute to biofilm formation (e.g., flagella, autotransporter proteins, fimbriae, curli, EPS, and F-type conjugative pilus) (10). Synthesis of bacterial surface appendages (including flagella) in early stages of biofilm formation allows motility and reversible attachment, as major determinants of biofilm structure. In the second stage, adhesive organelles, such as type I fimbriae, which are encoded by fim genes, and curli fimbriae, encoded by csg operon, contribute to biofilm formation for irreversible attachment. At this stage, flagella synthesis is repressed (11, 12).
Curli are wiry, long, and thin protein fibers on bacterial surfaces (13). They not only improve the binding potential to surfaces, such as polystyrene, glass cover slips, and stainless steel in some Enterohemorrhagic Escherichia coli (EHEC) strains, however, they also have a greater capacity to bind intestinal cells in comparison with non-curli-producing strains (14). In some previous studies, association of biofilm formation by extraintestinal E. coli and different adhesins such as curli has been reported (15). Various bacterial species are known to form biofilms, which bacterial biofilm formation is clearly preferred in the majority of nutrient-sufficient environments.
2. Objectives
This study examined the biofilm-producing ability of pathogenic strains of E. coli via microtiter-plate crystal violet method in two different media and investigated its correlation with csgA and fimA gene expression.
3. Methods
3.1. Pathogenic E. coli Isolation
E. coli was isolated from the different infections of cattle (metritis, mastitis, and urinary infection) in the Khuzestan Province. After culturing the samples on blood agar, they were incubated at 37°C for one day. Suspicious colonies were again streaked on blood agar and examined by oxidase test, catalase test, Gram staining, and biochemical tests (i.e., MacConkey, triple sugar iron agar, urease, sulfur-indole motility, phenylalanine deaminase, simmon citrate, lysine iron agar, and methyl red Voges-Proskauer tests) (16). Generally, E. coli is a normal flora of some organs in animals and humans. Therefore, identification of E. coli as the main pathogen in clinical samples is important.
3.2. Biofilm Assay
The modified technique described by Stepanovic et al. was used for the assessment of biofilm-producing ability on polystyrene microtiter plates (17). The positive control was E. coli ATCC 25922. After separately growing the isolates on Brain-Heart Infusion (BHI) broth with 1% sucrose (Merck, Germany) and Luria-Bertani (LB) broth medium, they were incubated for one day at 37°C. Then, 100 microliter (μL) of overnight cultures, were added to 1 milliliter (mL) of fresh BHI broth (1) containing 1% sucrose, as well as LB broth. Following that, 200 μL of bacterial suspension (0.5 MacFarland) was incubated in triplicate on sterile, flat-bottomed, polystyrene microtiter plates (96 wells; Maxwell, China). Sterile, supplemented BHI and LB broth were used as negative controls in triplicate.
After 24 hours of incubation at 37°C, microtiter plates were aspirated and washed by 300 μL/well of sterile normal saline three times. After drying, for fixation of probably forming biofilms, 200 μL of methanol was used for 15 minutes. Afterwards, methanol was removed and the plates were dried at ambient temperature. Biofilm staining was performed using 200 μL of crystal violet 2% (Hucker’s solution). After five minutes, washing with distilled water and drying at ambient temperature were done. An enzyme-linked immunosorbent assay (ELISA) reader (Biotek SX2, USA) was used to measure the absorbance at 600 nanometer (nm) after adding ethanol-acetone (discoloring solution; 200 μL) for 15 minutes.
For each strain, the arithmetic mean of optical density (OD) in three wells was compared with the mean absorbance of negative controls (ODnc). Biofilm formation was classified as follows: strong (4.ODnc < ODs); moderate (2.ODnc < ODs < 4.ODnc); poor (ODnc < ODs < 2.ODnc); and no production (ODs < ODnc) (17, 18).
3.3. Polymerase Chain Reaction (PCR) Assay
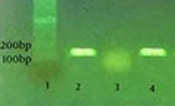
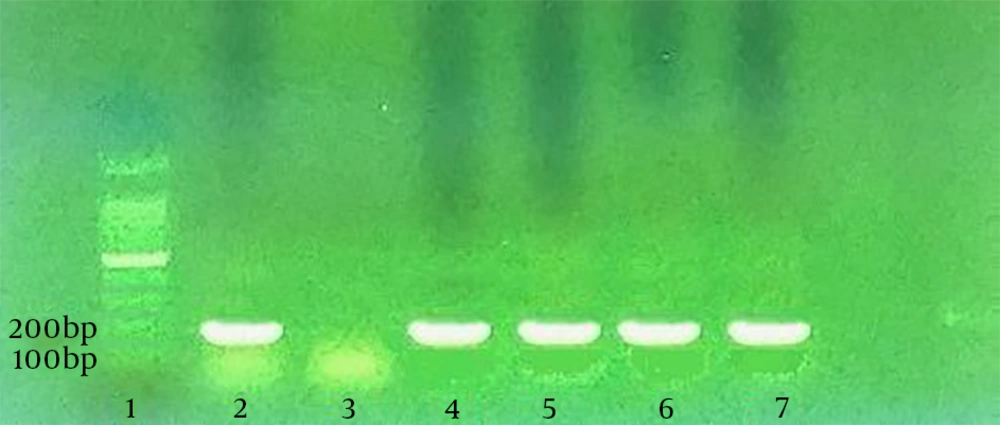
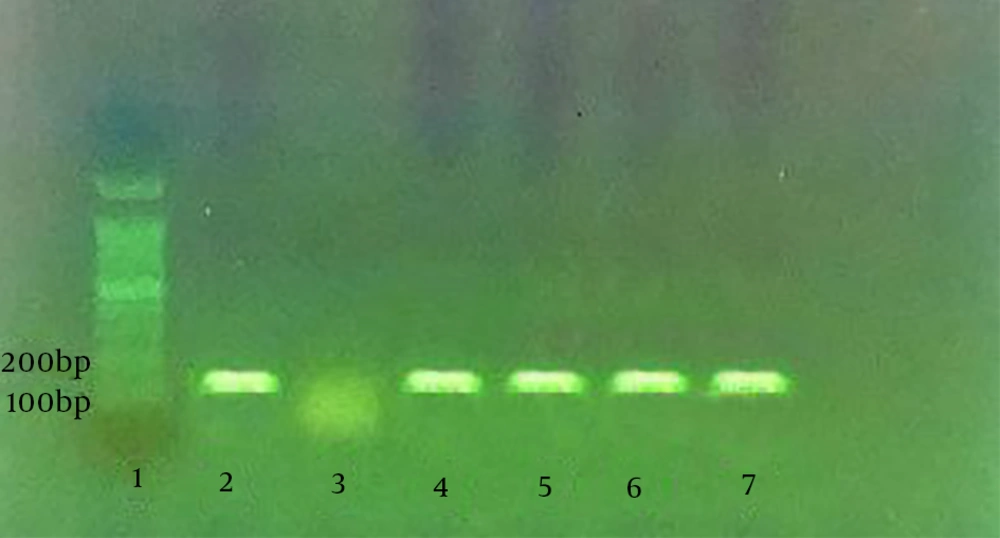
According to a study by Silva et al. PCR assay was performed to identify curli (csgA) and Type I fimbria (fimA) genes in the isolated E. coli. From each strain, DNA was first extracted through boiling the bacterial suspension in tris-EDTA (TE) buffer with 2-mercaptoethanol (2%). Following centrifugation, the supernatant of suspension was used as the DNA source. Silva et al. designed specific primers for fimA and csgA genes. The specific primers for csgA gene included 5’-ATCTGACCCAACGTGGCTTCG-3’ and 5’-GATGAGCGGTCGCGTTGTTACC-3’, which detected the 178-bp segment. The specific primers for fimA gene, which amplified the 119-bp segment, were 5’-CTCTGGCAATCGTTGTTCTGTCG-3’ and 5’-GCAAGCGGCGTTAACAACTTCC-3’.
The PCR assay was performed in a total volume of 25 μL, including bacterial DNA (5 μL), forward and reverse primers (1 μL; 10 pmol/L), 2X PCR Master Mix (12.5 μL; Ampliqon), and nuclease-free water (5.5 μL). The assay included a four-minute cycle at 94°C; followed by 30 cycles for 30 seconds at 94°C, for 30 seconds at 60°C, and for 30 seconds at 72°C, as well as a final four-minute extension at 72°C in a thermal cycler (Eppendorf, Germany). E. coli ATCC 25922 and nuclease-free water were used as positive and negative controls, respectively. Via electrophoresis on 1% agarose gel (Max Pure, Spain), the PCR products were visualized. Then, a UV transilluminator (UVtech, Germany) was used for staining (safe stain 1 × SinaClon) (19).
4. Results
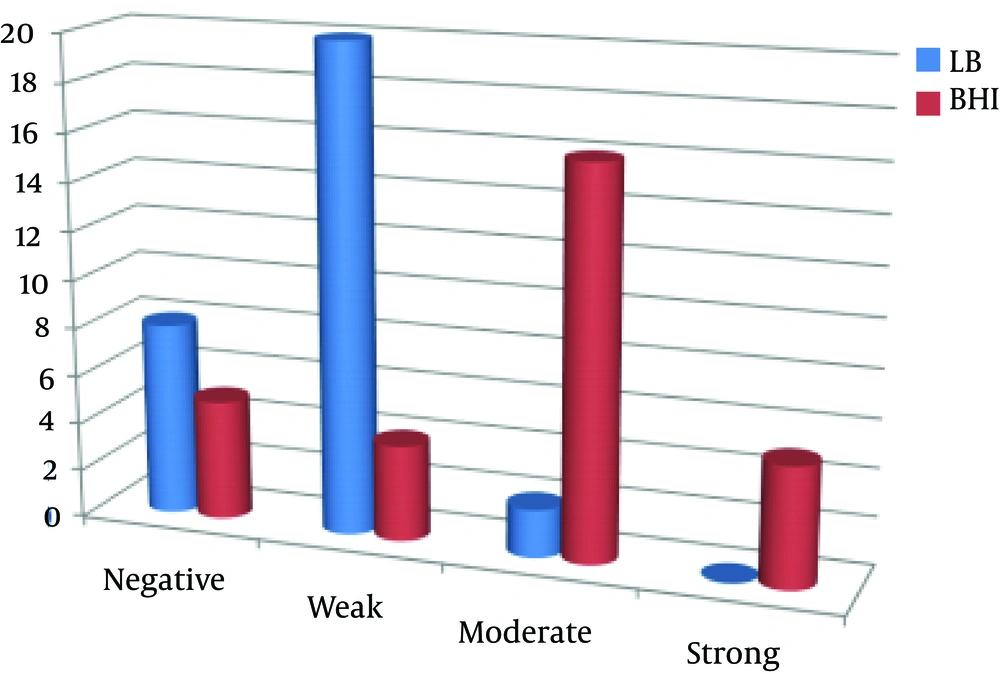
After collection of 30 E. coli isolates via cultivation of 64 clinical samples (mostly metritis samples; 83.3%), their biofilm-producing ability was evaluated using modified microtiter plates, presented by Stepanovic et al. Most isolates (66.6%) were poor biofilm producers in the LB medium, whereas in the BHI medium containing 1% sucrose, most isolates (53.3%) were moderate biofilm producers. In addition, the number of non-biofilm producing isolates was lower in the BHI medium (16.6%), compared to the LB medium (26.6%).
No isolate could strongly produce biofilm in the LB medium, while 16.6% of the isolates showed strong potentials. In fact, the BHI medium containing sucrose 1% had a greater biofilm-formation potential in comparison with the LB medium. Different levels of biofilm production by the studied media are demonstrated in the columns of Figure 1. According to the PCR assay, as demonstrated in Figures 2 and 3, all isolates (100%) were carriers of csgA gene, while only one isolate contained no fimA gene (3.3%) (Figures 2 and 3). Comparative diagram of the prevalence of studied genes is demonstrated in Diagram 1. In this research, all isolates with different biofilm-producing abilities contained csgA and fimA genes; therefore, the presence of csgA and fimA genes had no correlation with the biofilm-producing ability.
5. Discussion
Biofilm-producing bacteria cause various infections in humans and animals. Resistance of biofilm-producing bacteria to antibiotics and disinfectants is 500 - 5000 times higher than that of the planktonic type (20). Bacteria can be protected by the expression of specific resistance genes, besides the production of a large quantity of EPS during slow biofilm production (8). Dispersal of the planktonic type is required in new locations for biofilm production and colonization (21). The detected extraintestinal E. coli genotype may indicate the binding ability of E. coli strains to eukaryotic cells.
The improved binding ability of E. coli isolates to eukaryotic cells was indicated by the genotype of these extraintestinal isolates. Various surface organelles and extracellular molecules contribute to biofilm development of E. coli, including curli fimbriae, Type I pili, and flagella (22, 23). In several studies, correlation of different virulence factors in some bacteria with biofilm-producing ability was evaluated (24-27). Biofilm-producing ability has been examined by different quantification methods, including the commonly used microtiter plate systems (17, 28, 29). Biofilm formation by microtiter plate systems has been used for many different organisms and strains (18, 28, 30). Several studies have assessed the effects of enrichment medium type on biofilm assays. However, regulation of biofilm synthesis is a very complex process and there is scarce information in different species.
In this study, biofilm formation of 30 bovine extraintestinal E. coli isolates was assessed using the microtiter-plate crystal violet method in two culture media (LB and BHI + %1 sucrose), and correlation of attachment factors (Type 1 fimbria and curli fimbria) with biofilm-producing ability was examined in the isolates. By using two different culture media (LB and BHI + %1sucrose) in the microtiter plate system, 73.4% and 83.4% of the isolates were producers of biofilm in LB and BHI + %1 sucrose media, respectively.
The level of biofilm production was higher in the BHI medium containing sucrose (16.6%, strong production; 53.3%, moderate production) in comparison with the LB medium (0%, strong production; 66.6%, poor production). Some studies have examined the impact of enrichment medium type in biofilm assays. In this regard, Stepanovic et al. and Samet et al. found BHI medium to be superior to others (17, 31). In the study by Samet et al. BHI medium supplemented with 1% sucrose was used in biofilm production. In addition, no association was observed in uropathogenic E. coli strains between csgA and fimA genes.
Moori Bakhtiari and Javadmakoui in their studies detected no correlation between the biofilm-producing ability and presence of fimA and csgA genes in human uropathogenic E. coli strains. They recommended the BHI medium containing 1% sucrose for the study of biofilm-producing ability of these strains (32). In a study by Rijavec et al. biofilm production by pathogenic E. coli had no correlation with the presence of papC, usp, and sfa/foc virulence genes. However, Naves et al. showed that strong biofilm-producing strains of E. coli had a higher frequency of papG, sfa/foc, papC, hlyA, focG, and cnf1 genes (33, 34).
5.1. Conclusions
Since biofilm production is affected by different environmental factors, similar to the species and type of bacteria, extensive research is necessary on these isolates. In addition, considering the lack of association between the biofilm-producing ability and studied genes, evaluation of other involved genes is recommended.