1. Background
After the industrial revolution, fossil fuels became the main source of energy for humankind (1). Increased fossil fuel consumption has resulted in higher SOx, NOx, and CO2 emissions, leading to human health problems and global warming (2). In addition, the excess use of fossil fuels in the past decade has led this source of energy to depletion (3). Burning fossil fuels releases SO2, NO2, and other toxic substances such as formaldehyde and benzene, which all damage the human respiratory system and lungs and cause diseases like bronchitis, asthma, and emphysema (4, 5). The aforementioned problems give good reasons for research to find less harmful biofuels.
Until today, different kinds of biomass such as corn, sugar cane, soybean, coconut, jatropha, etc. have been used as feedstock to produce biofuels (6). These biomasses are known as the first and second biofuel generations, which cannot meet the worldwide demand because they have some challenges such as being also used as human food, high production cost, limited sources of land and water, production efficiency, and sustainable feedstock supply (7, 8). Algae (micro and macro), also known as the third biofuel generation, are considered a promising feedstock for the production of biofuels (9). The average photosynthesis efficiency of algae is higher than that of terrestrial plants (10). In addition, algae do not compete for land or human food resources, use solar energy, fix carbon dioxide from the atmosphere, and synthesize carbohydrates and lipids, which can be extracted for biofuel production (11).
Hydrothermal liquefaction (HTL) technique is the conversion route of wet biomass to bio-oil under subcritical conditions. HTL is preferred over pyrolysis in the conversion of biomass to bio-oil due to the lower reaction temperature (300°C - 375°C) and using water as the reaction medium, reactant solvent, and catalyst (12). Using water as the solvent and wet biomass as the feedstock are the main advantages of the HTL process in comparison with other conversion techniques, which decreases the conversion costs of drying (13). However, since HTL occurs in a subcritical water environment, the corrosion of equipment is one of the disadvantages of this process (14). In addition, in order to use bio-oil as a biofuel, catalytic upgrading is required (15).
Seaweeds can be classified into three taxonomic classes, including brown algae (Phaeophyta), green algae (Chlorophyta), and red algae (Rhodophyta) (16). Sargassum is a widespread brown macroalgae that could be found in the coastal areas, offshore platforms, and floating on seas; they also can be cultivated in artificial ponds. Compared to microalgae, macroalgae are considered interesting biomasses for biofuel production due to their low breeding cost and easy harvesting (17). Milledge and Harvey reported over 350 different species of Sargassum genus (18). In Iran, different Sargassum species such as Sargassum angustifolium, Sargassum boveanum, Sargassum fluitans, Sargassum latifolium, and Sargassum vulgare are found in the seashores of the Persian Gulf and the Oman Sea (19).
The HTL process can be conducted in the presence of a catalyst. The catalyst can reduce the retention time and operating temperature of the HTL process and it is commonly used to increase the bio-oil yield (1). Different catalysts have been employed by researchers to increase the bio-oil yield in the algae HTL process such as Na2CO3, zeolite, KOH, Ca(OH)2, etc. (20, 21). In this study, NiFe2O4 was employed as a nano-catalyst in order to study the catalytic effects on bio-oil production from Sargassum sp. To the best of our knowledge, no study has used NiFe2O4 as a catalyst in bio-oil production, especially algae bio-oil production. Rojas-Perez et al. (22) reported the production of bio-oil via the HTL process from Ulva fasciata by using Fe3O4 as the catalyst. The results showed a 9% increase in the bio-oil yield when catalyst loading increased to 1.25 wt%. Li et al. (10) studied bio-oil production from brown algae Sargassum patens, C.Agardh, via the HTL process. They found out that the maximum bio-oil yield occurred at 340°C. The obtained oil contained water, lipid, alcohol, phenol, esters, and aromatic compounds.
2. Objectives
In this study, the bio-oil production as a promising biofuel was studied by the HTL of Sargassum sp. in the presence of NiFe2O4 nanoparticles and analyzed in terms of the possibility of SOx and NOx emissions by CHNOS elemental analysis. In addition, gas chromatography-mass spectrometry (GC-MS) and Fourier transform infrared spectrometer (FTIR) analyses were conducted.
3. Methods
3.1. Materials
The algae sample of Sargassum sp. was collected in 2016 from the Persian Gulf, Iran. The algae were washed twice with distilled water to remove impurities. The algae were then dried in shade and pulverized to a particle size of < 0.5 mm.
3.2. Synthesis of Nanoparticles
Nickel (II) chloride hexahydrate (NiCl2.6H2O), Iron (III) chloride hexahydrate (FeCl3.6H2O), chloroform (CHCl3), and sodium hydroxide (NaOH) were purchased from a Merck subsidiary in Iran. The NiFe2O4 nanoparticles were synthesized via the co-precipitation method, as reported by Bagbi et al. (23) with some modifications. Briefly, according to a chemical equation (Equation 1), NiCl2.6H2O and FeCl3.6H2O with a molar ratio of 2:1 were mixed in deionized water under vigorous agitation at 60°C. Then, a solution of NaOH (1.5 M) was added dropwise into the solution. After 1 hour, NiFe2O4 was collected by a magnet and stored after being washed three times with distilled water. Maaz et al. (24) and Sagadevan et al. (25) synthesized nickel ferrite nanoparticles by the same materials and co-precipitation technique and confirmed the formation of NiFe2O4 nanoparticles by XRD and TEM (transmission electron microscopy) analyses. Since the synthesis methods are similar, it can be assumed that nanoparticles in this study had the same characteristics as reported in the mentioned studies.
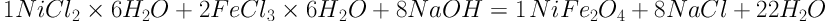
3.3. Experimental Methods
The HTL process occurs in subcritical water conditions (below 374°C and 22 MPa) (26). Mainly, the operating conditions for the biomass HTL process are 250°C - 350°C and 8 - 22 MPa (27-30). Hydrothermal carbonization occurs at a lower temperature (180°C - 250°C) and hydrothermal gasification in supercritical conditions of water, which both were not the aim of this study (31). In order to study the different HTL conditions, three temperature levels of 250°C, 300°C, and 350°C were chosen as experimental conditions.
The HTL of Sargassum sp. in the presence of nickel ferrite as a catalyst was conducted in a 75 mL cylindrical reactor. The reactor was loaded with 2.5 g of Sargassum sp. at a biomass-to-water ratio of 1:10. Then, NiFe2O4 was loaded into the reactor with the catalyst-to-biomass ratio of 2:10 (dry basis). After that, distilled water was added to the reactor. The mixture was then purged under N2 atmosphere for one minute. The reactor was inserted into a furnace and heated to the desired temperature (250°C, 300°C, and 350°C). The temperature was held constant for 35 minutes. The measurements showed that the reactor would reach the set reaction temperature after about 10 minutes at a heating rate of 25 - 35°C/min. The reactor was then removed from the furnace and put into a cool-water bath to reach the room temperature.
The reactors were opened carefully and an equal amount of distilled water and chloroform was added to the reactor to completely recover the content. Then, the liquid phase was filtered using vacuum filtration to separate the solid phase. Next, chloroform was removed from the chloroform soluble liquid to obtain the bio-oil. The bio-oil yield was calculated using Equation 2 (32):
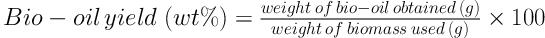
3.4. Analysis Methods
The elemental composition of the product was determined using a “Flash EA1112” CHNOS analyzer at the Central Instrumental Lab, University of Tehran, Iran. The components of the bio-oil were analyzed by the GC-MS technique. The GC-MS analysis was carried out with an Agilent 19091S-433 analyzer equipped with an HP-5MS column at Kimiazi Lab, Tehran, Iran. The helium carrier gas was set at a rate of 22.9 milliliters per minute. The injector temperature was set to 300°C and 2.0 microliters of bio-oil/methanol solution were injected into the GC-MS apparatus using a syringe. The NIST mass spectral library was used to identify the bio-oil compounds. In addition, an FTIR spectrometer was employed to determine the functional groups of the bio-oil.
4. Results
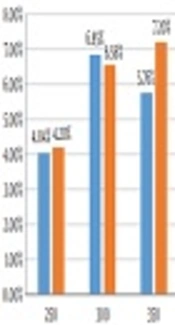
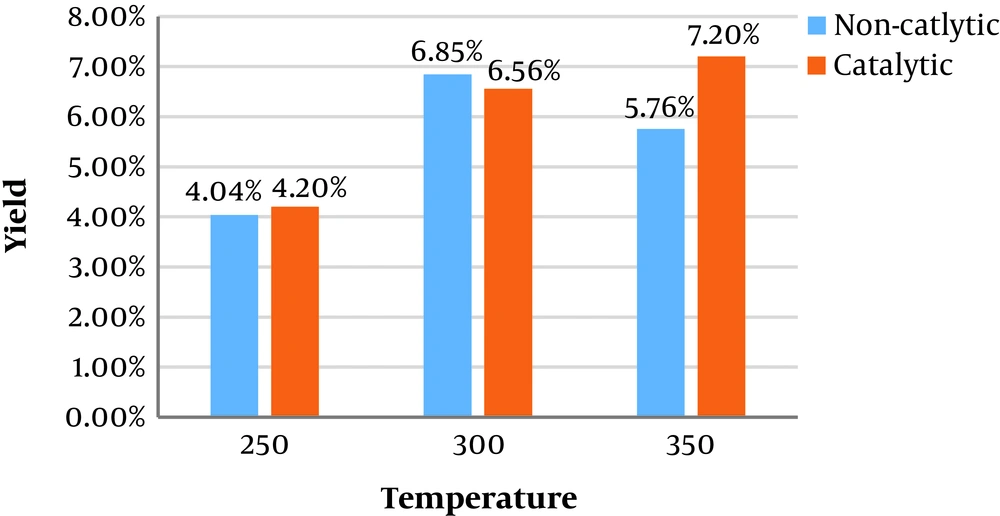
In this study, different amounts of catalysts were synthesized via the co-precipitation method to evaluate the results of using NiFe2O4 nanoparticles on the bio-oil yield of Sargassum sp. Figure 1 illustrates catalytic and non-catalytic bio-oil yield of Sargassum sp. at different temperatures. The yield of bio-oil was calculated on dry weight basis. As it is shown, the maximum bio-oil yield without a catalyst is 6.85 wt% at 300°C, while the maximum bio-oil yield of 7.20 wt% was achieved at the temperature of 350°C. In non-catalytic HTL, at the beginning the bio-oil yield increased by rising the temperature from 250 to 300°C and then decreased from 300 - 350°C.
4.1. Analysis of Bio-Oil
The elemental structure of bio-oil inspected by CHNOS elemental analysis in terms of carbon, hydrogen, nitrogen, sulfur and oxygen contents. The GC-MS and FT-IR analysis were employed to recognize the components and identify the functional group of bio-oil.
4.2. Elemental Analysis
The elemental analysis of obtained bio-oils shows an increase in the carbon content from 68.34 to 78.23 followed by an increase in hydrogen content from 8.91 to 10.15%. The content of oxygen, nitrogen, and sulfur was about 10%, 4%, and zero respectively.
4.3. GC-MS Analysis
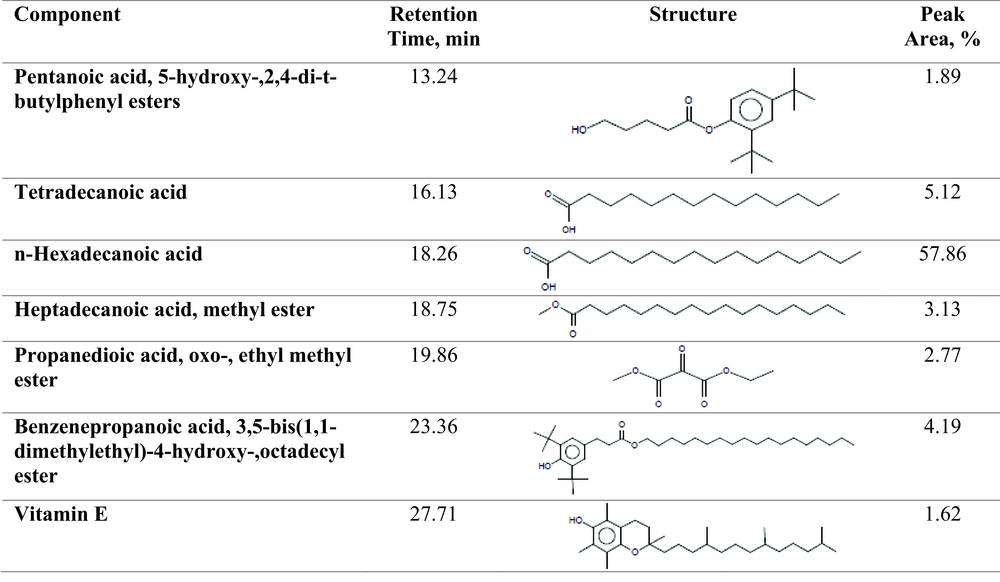
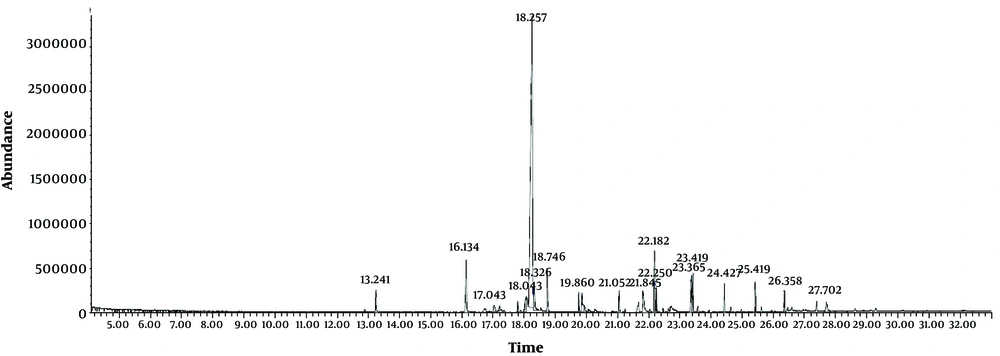
GC-MS analysis was used in order to study the chemical component of Sargassum bio-oil. The chromatogram was analyzed using a NIST library. The main components of the bio oil is defined as the proportion of the area under the peak of the curve to the total area of the peaks in the chromatograms. As demonstrated in Figure 2, the largest bio-oil peak area at the retention time of 18.257 belongs to n-Hexadecanoic acid (palmitic acid) (57.86%) followed by tetradecanoic acid (5.12%) at the retention time of 16.13 (Figure 3).
4.4. FTIR Analysis
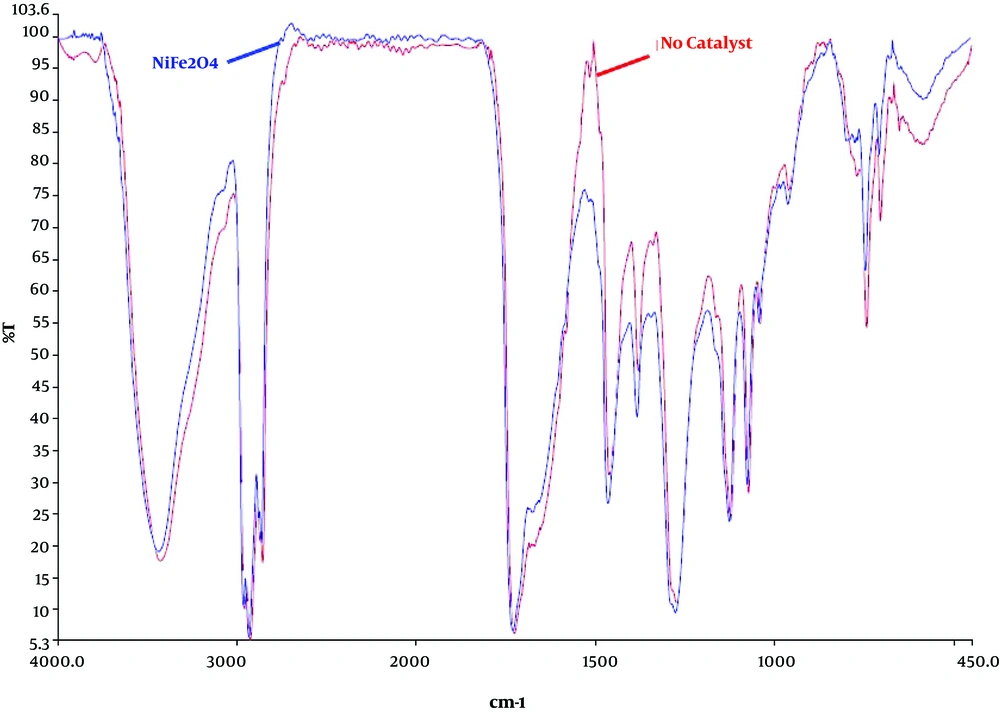
FTIR spectra of the bio-oil obtained in non-catalyst and catalytic HTL are shown in Figure 4. As it is shown both spectra exhibit similar vibrational mode.
The strong absorption at around 3420 cm-1 is possibly from O-H or N-H stretching vibration, it could be from water in the bio-oil. The absorptions at 2960 - 2855 cm-1 are related to C-H bonding stretching vibration, indicating alkyl C-H. Lots of weak peaks at 2100 to 2260 cm-1 represents the alkyne CC bonding stretching vibration. The strong C=O stretching vibration at 1726 cm-1 shows the presence of aldehydes, esters, ketones, or acids. The medium peak at around 1600 cm-1 shows the C=C bonding stretching vibration. The bands peaking around 1350 to 1450 cm-1 indicates the C-H bending characteristic of fats. The strong peak at 1100 to 1300 cm-1 shows the alcoholic C-O bonding stretching vibration. And the peaks occurring at around 700 cm-1 represent the presence of aromatic compounds (33).
5. Discussion
A low bio-oil yield was expected due to using macro-algae as feedstock of HTL, which has also been reported by other researchers. Parsa et al. (27) reported a bio-crude yield of 15.7 and 16.9 percent for Gracilaria gracilis and Cladophora glomerata macro-algae, respectively. Generally, macro-algae species have lower lipid content and HHV in comparison to microalgae species, but some factors such as easy harvesting, the capability of cultivation in natural environments, lower initial capital, fast growing, and high biomass productivity, made macro-algae an interest feedstock for bio-oil production (2, 34, 35). The increase in bio-oil yield is possible by changing other liquefaction parameters such as process time, biomass-to-water ratio, etc.
In non-catalytic HTL, decrease in the bio-oil yield could be attributed to higher conversion of compounds to the gas phase or secondary decomposition of compounds into lighter molecules. The bio-oil yield for catalytic conversion increased by rising the temperature from 250 to 350. This could happen due to an increase in hydrolysis reactions of biomass in response to temperature rise. At subcritical conditions, the energy required for depolymerization, isomerization and repolymerization reactions provided due to increasing the temperature of the process and more bio-oil achieves (36). It seems that NiFe2O4 prevent secondary decomposition and conversion of bio-oil to the gas phase while the temperature increases to 350, compared to the non-catalytic process.
Elemental analysis shows that the content of Oxygen, nitrogen and sulfur was about 10%, 4%, and zero respectively. Presence of nitrogen and sulfur leads to NOx and SOx emission in the combustion of bio-oil, while the presence of oxygen reduces the heating value of bio-oil (27). So, the content of oxygen, nitrogen, and sulfur must be reduced to zero by using catalytic processes. Díaz-Vázquez et al. (37) reported similar elemental content for Sargassum with 71.54% carbon, 8.05% hydrogen, 18.05 oxygen, and low nitrogen and sulfur content.
GC-MS analysis results shows the majority of n-hexadecanoic acid. Other researchers also indicated that the main fatty-acid in a large number of macro-algae such as Sargassum species, Dictyota dichotoma, Schizochytrium limacinum, etc. is n-hexadecanoic acid (18, 38). Anand et al. (39) and Li (40) reported that the majority of n-Hexadecanoic acid and Tetradecanoic acid in bio-oil were obtained from pyrolysis of Schizochytrium limacinum microalga. The GC-MS results are consistent with the FTIR results.
5.1. Conclusions
In this paper, the HTL was performed on Sargassum sp. macroalgae at three temperature levels of 250°C, 300°C, and 350°C, with and without using NiFe22O4 as a catalyst. The liquefaction yield increased by increasing the reaction temperature from 250°C to 300°C for both processes, but for the non-catalytic process, the bio-oil yield decreased when the temperature increased from 300°C to 350°C. The obtained bio-oil had no sulfur with low nitrogen content. The N2 content could be reduced to zero by upgrading the bio-oil; thus, the bio-oil would be healthier and more environmentally friendly than fossil fuels due to zero SOx and NOx emissions. GC-MS and FTIR analyses were conducted to identify the compounds and the functional groups of the bio-oil.