1. Background
Today, the prevalence of obesity is seen as a major social challenge in various areas of health, especially in the public health sector (1). Obesity is one of the key risk factors in a number of diseases, such as cardiovascular disease, diabetes, high blood pressure, metabolic disorders, and various types of cancer (2). Obese people are more susceptible to risk as well as chronic and endemic diseases, and ultimately these people face a reduction in life expectancy and early death. Sedentary lifestyle, unhealthy diet, obesity, overweight, and unknown genetic factors are factors associated with metabolic syndrome (3). In this vein, metabolic syndrome is one of the most common and rising diseases in the world and its basic causes can be attributed to obesity, genetic factors, and physical inactivity. The metabolic syndrome is closely related to the metabolic disorder of insulin resistance, in which the sensitivity of the tissues of the body to the normal functioning of insulin is impaired. Insulin resistance is a path physiologic disorder that occurs due to a defect in the pathways through which insulin stimulates glucose uptake (4).
On the other hand, fat mass gain in obese people is accompanied by a change in the secretion of adipokines (5). It is now believed that macrophages absorbed from the bloodstream into adipose tissue are the main source of inflammation in obesity. Minor chronic inflammation is associated with higher than normal levels of several cytokines, including alpha tumor necrotizing factor (TNF-α), interleukin 6 (IL-6), and C-reactive protein (CRP) (6). Evidence suggests that levels of inflammatory markers are high in obesity, and a high correlation exists between these inflammatory markers and metabolic-heart problems. The information suggests that inflammation could play a key function in the pathogenesis of metabolic syndrome (5). In recent years, C-reactive protein, as an inflammatory agent, can directly contribute to the pathogenesis of atherosclerosis. Although this substance is produced in the liver, new research has shown that it can be built in the intimal layer of the artery with atherosclerosis as well. Apart from the role of the inflammatory marker, CRP can cause vasodilatation by various mechanisms, such as decreasing nitric oxide production (NO), increasing molecular adhesion, and altering low-density lipoprotein (LDL) adsorption by macrophages. Increasing this protein as the most sensitive inflammatory marker and independent anticipatory indicator of cardiovascular risk rises the risk of cardiovascular events by two to five times (7). It may also be part of the inflammation caused by the accumulation of fat mass and lipid profiles due to undesirable oxidative stress and peroxidation of lipids, especially as it affects the fat deposits in the arteries. Oxidized LDL cholesterol (Ox-LDL) is considered as one of the most important factors in initiating and accelerating vascular fat accumulation and sedimentation (8). Studies have shown that Ox-LDL’s atherogenic property is greater than low-density lipoprotein cholesterol (LDL-C). Low-density lipoprotein oxidation stimulates monocytes, followed by accumulation of vascular macrophages, which are afflicted with arterial cramps (9, 10). In fact, the formation of Ox-LDL from LDL indicates a rise in oxidative stress that ultimately results in the destruction of endothelium tissue with the participation of free radicals, and, together with the progression of atherosclerotic lesions, in particular, the precipitation of cholesterol, and the creation of plaque reduces the vascular function against inflammation. Given the prevalence of obesity and the rapid progression of this complication in the population, research has focused on regulating and balancing body weight, therefore, it is necessary to pay more attention to this problem and provide cost-effective and affordable ways for it to be identified and presented. In this regard, encouraging participation in sports activities is an appropriate and cost-effective option, playing a pivotal role in enhancing the health of the community and, to some extent, managing metabolic diseases. Therefore, exercise and sport exercises are one of the important interventions in preventing and controlling obesity and associated metabolic factors.
Research reports reveal contradictory findings, in which some researchers find that regular physical activity is effective in reducing oxidative stress, especially Ox-LDL levels, and consequently effective prevention of atherosclerosis (11). However, it has been reported that high-intensity short-term exercising maximizes oxidative stress, which interferes with the equilibrium between reactive oxygen species (ROS) and antioxidant defense, which can affect the entire body (12). By contrast, regular aerobic exercise and moderate-intensity exercise increases production and release of antioxidants (13). With regard to the above points, the researcher is addressing the question of how much exercise protocol (aerobic practice) can reduce Ox-LDL and changes in blood lipoproteins. Thus, this study was aimed at investigating the impact of eight-week aerobic exercises on Ox-LDL levels and cardiovascular risk factors among obese and overweight women.
2. Objectives
Obesity and being overweight has become a public health problem over the past 20 years. It has been attributed to many social and environmental determinants. Several studies have linked in this regard; therefore one of the best strategies for preventing obesity and its associated inflammation is participation in regular physical activity. This study was aimed at evaluating the impact of eight weeks of aerobic exercises on the levels of Ox-LDL and cardiovascular risk factors among obese and overweight females.
3. Methods
This study comprised a quasi-experimental design involving a pretest-posttest with the control group. The statistical population of this study included obese and overweight women in 2018 in Gachsaran city. Following a public announcement for participation in this study, those who fulfilled the inclusion criteria, namely not participating in regular sports activities during the past six months, lack of metabolic diseases, lack of alcohol and cigarettes use, and absence of chronic diseases such as cardiovascular and joint diseases, were selected. Then, 22 eligible obese and overweight women aged 35 to 45 years were selected randomly and placed in two groups of experimental (exercise) and control (11). After completing the consent form and physical activities readiness questionnaire (PAR-Q), subjects were evaluated for physical composition measurements.
3.1. Body Composition and Blood Measurements
The height of the subjects was measured without shoes using a height gauge. Measuring people’s weight was made with light clothing, no shoes, and a digital scale of the Seca model (made in Germany). Body mass index (BMI) was measured by dividing the weight (in kilograms) to the square of height (in meters).
Fasting and resting morning blood samples (10 mL) were obtained at the same time preceding and following eight-week treatment. The participants were required to fast at least for half a day, and the fasting blood samples were collected by venipuncture. The Ox-LDL level was determined in duplicate by utilizing enzyme-linked immunosorbent assay (ELISA) kits (Mercodia, Uppsala, Sweden). High-sensitivity C-reactive protein (Hs-CRP) levels were measured in duplicate using enzyme-linked immunosorbent assay (ELISA) kits (Diagnostics Brochem Canada, Inc with a sensitivity 10 ng/mL). Plasma glucose level was measured through the enzymatic (GOD-PAP, Glucose Oxidase-Amino Antipyrine) colorimetric method (Pars Azmoun, Tehran, Iran). The intra and inter-assay coefficients of variation of glucose were found to be < 1.3% with a sensitivity of 1 mg/dl. The serum insulin level was calculated via radioimmunoassay (RIA), and the insulin resistance index was gauged based on the homeostasis model assessment (HOMA-IR). The findings were well consistent with the hyperinsulinemic-euglycemic clamp in diabetic people (5). Automated techniques were used to assay serum cholesterol triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
3.2. Exercise Training
The subjects receiving aerobic training attended four exercise training sessions for a period of eight weeks on a weekly basis. The training included a five to 10 minute warm-up followed by muscle stretches. Moreover, it constituted slow-pace jogging and walking at 50% - 55% of the maximal heart rate (HRmax) for 30 - 45 min each day for three days per week and lasted eight weeks. The training involved 30 minutes of walking at the initial sessions and was increased to 45 minutes each session until the end of training. Every exercising session closed with some cooling down activities. The intensity of the exercises were curbed by the researchers via a heart rate monitor in order to ascertain that the heart rate was maintained at the range of 50% and 60% of HRmax during the experiment.
3.3. Statistical Analysis
All data were analyzed through SPSS (version 21). Mean ± and standard deviation (SD) were identified. Independent samples t-test and paired t-test were employed to evaluate shifts among the variables of the study. P values less than 0.05 were considered statistically significant.
4. Results
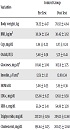
The results demonstrated that eight weeks of aerobic exercise resulted in a significant reduction in body mass index (t = 5.24 and P = 0.012) in the experimental group. However, these changes were insignificant in the case of the control group. The mean and standard deviation of the variables in the pre-test and post-test are presented in Table 1. The results of independent samples t-test indicated a significant discrepancy in the pre-test and post-test of the experimental group for the main variables including insulin resistance, Hs-CRP. Nonetheless, no significant difference was found in the rate of Ox-LDL after eight weeks of aerobic training. The results of independent t-test on insulin resistance (t = 3.48 and P = 0.002) and Hs-CRP (t = 17.204 and P = 0.001) index variables showed a significant difference between the experimental and control groups after eight weeks of exercise training, however, there was no significant difference in Ox-LDL index (t = 9.27 and P = 0.07).
| Variables | Control Group | Training Group | t | P Value | ||
|---|---|---|---|---|---|---|
| Pre Test | Post Test | Pre Test | Post Test | |||
| Body weight, kg | 78.25 ± 4.47 | 78.92 ± 4.64 | 78.08 ± 4.52 | 76.01 ± 4.65 | 12.46 | 0.001 |
| BMI, kg/m2 | 30.24 ± 3.54 | 30.45 ± 3.62 | 30.42 ± 2.36 | 28.68 ± 1.8 | 5.24 | 0.012 |
| CRP, mg/dL | 1.84 ± 0.23 | 1.87 ± 0.24 | 1.85 ± .25 | 1.53 ± .23 | 17.204 | 0.001 |
| Ox ldl, IU/L | 5.68 ± 0.38 | 5.71 ± 0.41 | 5.75 ± 0.31 | 5.68 ± 0.27 | 9.27 | 0.07 |
| Glucoses, mg/dL | 101.41 ± 3.06 | 102.11 ± 3.75 | 100.70 ± 6.4 | 97.81 ± 5.43 | 8.36 | 0.001 |
| Insulin, μU/mL | 12.51 ± 1.12 | 12.80.1.34 | 12.43 ± 1.17 | 11.90 ± 1.31 | 6.23 | 0.001 |
| HOMA-IR | 3.08 ± 0.35 | 3.19 ± 0.36 | 3.09 ± 0.39 | 2.87 ± 0.32 | 3.48 | 0.002 |
| LDL-c, mg/dL | 125.66 ± 8.27 | 126.11 ± 9.58 | 126.2 ± 8.4 | 119.40 ± 8.21 | 7.30 | 0.001 |
| HDL-c, mg/dL | 55.24 ± 3.41 | 54.86 ± 3.28 | 54.73 ± 2.83 | 57.266 ± 2.71 | -4.47 | 0.001 |
| Triglyceride, mg/dL | 207.20 ± 11.56 | 209.21 ± 12.68 | 206.95 ± 10.47 | 191.86 ± 8.62 | 9.22 | 0.001 |
| Cholesterol, mg/dL | 198.64 ± 11.25 | 200.23 ± 12.68 | 198.60 ± 12.54 | 186.06 ± 10.68 | 9.44 | 0.001 |
5. Discussion
In the present study, the effects of aerobic exercises on blood levels of Ox-LDL and insulin resistance, CRP and blood lipoprotein were evaluated in obese and overweight women. Based on the findings, Ox-LDL levels did not change significantly after eight weeks of aerobic exercises. It seems that this type of exercise does not have enough stimulation for effective mechanisms to reduce Ox-LDL in obese women. The findings of some researchers showed that the Ox-LDL after a period of aerobic exercise combined with diet had a significant reduction in coronary heart disease (14). On the other hand, Rahimi and Jalili (15), showed that Ox-LDL concentration immediately decreased after resistance training with green tea consumption. Zahabi et al. (16), in their research showed that four weeks of combined exercise leads to inhibition of oxidation of LDL and consequently prevents cardiovascular disease. Fathei et el. (17), demonstrated that eight weeks of aerobic training, green tea consumption, and their composition reduce the cardiovascular risk factors in inactive obese women in a relatively similar manner. The research evidence showed the association between Ox-LDL and obesity (18, 19), which contradicts the findings of this study. Perhaps the main reasons for this discrepancy between the findings may be the manner and nature of the different exercises in these studies (in this study, the intensity of training was moderate). On the other hand, the findings of the study conducted by Afzalpour et al. (20), showed that Ox-LDL levels did not change significantly after eight weeks of aerobic training, which is consistent with this study. It has been shown that obesity is associated with increased oxidative stress and antioxidant defense in resting conditions. Therefore, since obese people have lower antioxidant defense than normal people, they are more exposed to free radicals.
Due to exercise activity, the amount of antioxidants increases and prevents the processes leading to the production of Ox-LDL, so the body’s immune system improves due to increased antioxidants due to exercise activity (21). In obese people, there are also higher levels of lipid peroxidation (such as cholesterol and triglyceride). Higher fat levels in obese individuals stimulate the production of free radicals in cells more than normal (22), which may lead to damage to the protein, lipid, and DNA of the cell. Sport exercises prevent the accumulation of fatty acids in muscle cells by increasing the oxidation of fatty acids. Hence, lifestyle changes focusing on weight loss and increased physical activity are the main strategies for coping with the development of obesity (23). Based on the findings of this study, after eight weeks of aerobic exercises, blood CRP showed a significant decrease compared to baseline. CRP is known as the independent marker of cardiovascular complications, whose levels rapidly increase in response to inflammation in blood circulation and contribute to the pathogenesis of chronic inflammatory diseases such as cardiovascular disease, diabetes, and cancer (24); and one of the most important drivers of its production is obesity (25). Research reports reveal contradictory results regardin the effect of regular physical exercises on blood pressure levels in CRP. Eizadi et al. (26), observed significant reductions in CRP values after three months of aerobic exercise. On the other hand, in Wong et al. (27), despite a significant reduction in body mass index and an increase in fat-free mass, CRP concentration did not significantly decrease.
Previous studies on the effect of exercise on CRP have given different results. Lakka et al. (28), reported a significant reduction in serum CRP levels of inactive people after the 20-week cycling exercise (30 to 50 minutes per week). In addition, Olson et al. (29), reported improvement in hs-CRP levels after 12 months of exercise in obese women, while Wang et al. (27), did not see any significant change in CRP levels after three months of regular exercise in obese subjects.
On the other hand, the findings of this study showed that there was a significant relationship between training practices and insulin resistance. Although insulin is one of the most important components that are effective in insulin resistance, insulin response to exercise may be different. Eizadi et al. (26), reported that short-term exercise training does not improve insulin sensitivity or may worsen it. Other researchers found that long-term endurance (aerobic) exercises reduced insulin sensitivity (30), which is consistent with the findings of the present study. The causes of insulin resistance are reversible with weight loss, diet, and physical activity. Therefore, sports exercises can increase the body’s response to insulin through increased glucose transport to muscle cells (GLUT-4) and insulin receptor substrates (IRS), as well as muscle mass increase (more than 75% glucose uptake is due to insulin-induced muscle tissue stimulation) (31). The findings of this study showed that aerobic exercises improved blood lipoproteins including decreased total glycerides (TG), total cholesterol (TC), LDL, and increased HDL-C. Therefore, due to the fact that LDL oxidation, by creating fatty plaques in the endothelial wall, is an important event in atherogenesis, improving HDL-C can have a protective effect against LDL oxidation. Researchers have found that continuous physical training can lead to an increase in HDL-C over many years of life. It is worth mentioning that lipoprotein lipase enzyme is activated by physical activity, and the more it is activated, the more amount of HDL-C is formed. On the other hand, increased production of HDL-C, due to physical activity, has been reported in addition to the activation of lipoprotein lipase due to the activation of lecithin-cholesterol-acyl transferase and decreased liver lipase activity. It has been argued that HDL acts as an antioxidant in addition to reversing the transfusion of cholesterol from tissue to the liver and preventing the accumulation of cholesterol in the tissue. In addition, by having the paraoxonase enzyme, it prevents further oxidation of LDL, and in this regard, physical activity plays a strong protective role for the body (32).
5.1. Conclusions
The results of this study showed that eight weeks of aerobic exercise improved cardiovascular risk factors and inflammatory factors, which result in the improvement in insulin resistance and blood lipoprotein resistance. Therefore, it seems that this practice can be recommended for adult women who are obese and overweight. So far, many studies have not been done regarding the effects of aerobic exercise on the Ox-LDL index. Meanwhile, the findings of this study should be interpreted with some caution owing to lack of control over some factors such as the participants’ genetic tendencies regarding cellular modifications and adaptation, motivation level, psychological stress, and even lifestyle. Therefore, other studies are warranted to probe further into varying intensities of exercising with longer intervention periods to provide a precise understanding of the appropriate intensity of exercise for weight gain or loss.