1. Background
Urinary tract infections (UTIs) are the most common infections in the community and in hospitalized patients that affect 150 million people each year worldwide with annual health care costs of more than 6 billion dollars (1). UTI is a significant obstacle in low-income countries due to financial consequences related to health care costs. UTIs were clinically classified into uncomplicated UTIs that typically affect individuals who have no structural or functional urinary tract abnormalities and complicated UTIs that are associated with factors that compromise the urinary tract or host defense (2). Although the etiology of UTIs is different from country to country, the most common pathogens of UTIs are Gram-negative bacteria, such as Escherichia coli, Klebsiella species, Proteus species, Pseudomonas aeruginosa, Enterobacter species, Acinetobacter species, and Citrobacter species. Gram-positive bacteria associated with UTI include Staphylococcus saprophyticus, Enterococcus species, and coagulase-negative Staphylococcus (3). Different factors, such as age, sex, comorbid disease, type of pathogen, and site of the infection (lower or upper urinary tract involvement) can influence the treatment of UTIs (4).
The growth of antimicrobial resistance and increasing the proportion of multidrug-resistant (MDR) and extensively drug-resistant (XDR) pathogens in UTI are associated with higher rates of inadequate antibiotic coverage and impaired empirical therapy (5). Although UTIs can lead to irreversible kidney injury and increase the risk of bacteremia, most UTIs are not life-threatening (6). Mohamed et al. (7) conducted the first study on UTI in Somalia. The limitation of their study was a limited focus on a single uropathogen as well as the limited number of antimicrobial susceptibility profiles and the small number of studied patients. This study was performed due to the significance of this topic in Somalia and because of the overuse of antibiotics resulting from the lack of national policies.
2. Objectives
This study investigated the overall prevalence of UTIs in children, adults, and pregnant cases, and also uropathogens and comorbidities associated with UTIs, antibiotic sensitivity, and resistance pattern against all uropathogens was assessed. Also, we aimed at determining the most appropriate empirical antibiotics to treat UTIs in the community and hospitalized patients.
3. Methods
This retrospective study was carried out on a total of 2,485 urine cultures performed between January and December 2019 at Mogadishu Somali Turkish Training and Research Hospital. The structured data of these patients were analyzed retrospectively. Patients in all age groups with the hospital- and community-acquired UTI with a positive urine culture visiting the hospital included in the study. The clean-catch midstream urine samples were collected from the patients who had a suspected UTI in well-preserved containers and transferred promptly to the microbiology and laboratory unit.
Through the standard Kirby-Bauer disk diffusion method and using commercial disks, antimicrobial sensitivity and resistance were determined based on the Clinical and Laboratory Standards Institute (CLSI) system (8, 9). Definite UTI pathogen was defined as a single pathogen with adequate colony formation unit (CFU) (i.e. > 100,000 CFU/mL in voiding urine; > 10,000 CFU/mL in catheterized urine; and > 1000 CFU/mL in suprapubic puncture) in one urine culture (10). The identification of the microorganisms was done using eosin methylene blue agar (EMB) and blood agar. Mueller-Hinton agar was used to assess antimicrobial sensitivity and resistance. Antimicrobial sensitivity and resistance of Enterococcus species were measured using blood agar.
The antibiotic susceptibility of uropathogens was studied against piperacillin/tazobactam (100/10 mcg), meropenem (10 mcg), ertapenem (10 mcg), colistin (10 mcg), amikacin (30 mcg), tigecycline (15 µg), cefepime (30 µg), cefazolin (30 µg), ceftazidime (30 µg), piperacillin (100 µg), linezolid (30 mcg), clindamycin (2 mcg), penicillin (G 1U), trimethoprim/sulfamethoxazole (1.25/23.75 mcg), vancomycin (30 mcg), daptomycin (30 mcg), tetracycline (30 mcg), erythromycin (15 mcg), cefoxitin (30 mcg), ciprofloxacin (5 mcg), nitrofurantoin (300 mcg), and teicoplanin (30 µg). The study was conducted on all 361 patients whose urine cultures showed growth. The studied parameters included age, sex, comorbid diseases, microbiological urine culture results, antimicrobial sensitivity, and resistance patterns. The authors focused on the spectrum of MDR pathogens throughout the study. Multidrug-resistant microorganism is a pathogen that is resistant to two or more antimicrobial agents.
An antibiogram of 35 distinct antibiotics in varying categories was performed during the study period in the microbiology and laboratory unit of the hospital. The univariate descriptive study design was used to analyze the analytic parameters using IBM SPSS Statistics 23 version, and the results were expressed as percentages. To detect the significant association between the variables, the chi-square test and cross-tabulations were used.
4. Results
A total of 2,485 urine cultures were performed in one year at the Laboratory and Microbiology Unit of the hospital considering the standard guidelines. As cited in the previous studies, a slight predominance of females was found constituted 51% of the total patients, while 49% of the participants were male. Most of the participants (57.1%) had community-acquired UTI, and an increasing antimicrobial resistance spectrum in these patients was noticed; however, the rate of nosocomial-acquired UTI was relatively close to the community-acquired UTI, which accounted for 42.9% of the total patients. Only about two-thirds of the cases (65%) had complicated UTI either through structural or functional abnormalities. Of these, renal failure was the most common comorbidity of the patients (23.5%) followed by diabetes in 13.3%, and urinary stone of varying locations in 10% of the patients.
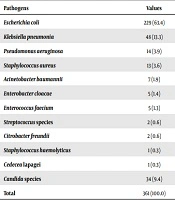
Twelve uropathogens showed growth in the culture, eleven bacterial uropathogens, and candida. Escherichia coli was the main uropathogen identified from the cultures (about 63.4% of the total cases: two-thirds), followed by Klebsiella pneumoniae found in 13.3% of the patients. Pseudomonas aeruginosa, S. aureus, A. baumannii, Enterobacter cloacae, and Enterococcus faecium also showed growth as cited in Table 1. According to the age groups, the 19 - 59 and > 60 years age groups included the most number of the patients accounting for 44% of each group, followed by the newborn to 28 years age group accounting for 12% of the cases. Escherichia coli was the most predominant pathogen in all age categories, in both genders and in the community- and hospital-acquired UTIs. An antibiogram of 35 distinct antibiotics in varying categories was performed during the study period in the microbiology and laboratory unit of the hospital.
| Pathogens | Values |
|---|---|
| Escherichia coli | 229 (63.4) |
| Klebsiella pneumonia | 48 (13.3) |
| Pseudomonas aeruginosa | 14 (3.9) |
| Staphylococcus aureus | 13 (3.6) |
| Acinetobacter baumannii | 7 (1.9) |
| Enterobacter cloacae | 5 (1.4) |
| Enterococcus faecium | 5 (1.3) |
| Streptococcus species | 2 (0.6) |
| Citrobacter freundii | 2 (0.6) |
| Staphylococcus haemolyticus | 1 (0.3) |
| Cedecea lapagei | 1 (0.3) |
| Candida species | 34 (9.4) |
| Total | 361 (100.0) |
aValues are expressed as No. (%).
Ceftriaxone, trimethoprim/sulfamethoxazole (TMP/SMX), ampicillin, cefuroxime, cefixime, cephazolin, and cefepime revealed the highest resistance level (about 82% - 100%) against uropathogens regardless of a specific pathogen (Table 2). Besides, fluoroquinolones (ciprofloxacin in 67.7% and levofloxacin in 54.2%) divulged increasing resistance rates against uropathogens. Tigecycline, colimycin, vancomycin, linezolid, and teicoplanin were found with the most powerful sensitivity rate among all microbes; in about 100% of the cases. Moreover, fosfomycin, nitrofurantoin, and amikacin manifested a significant sensitivity rate ranging from 86% - 95% against microorganisms. We investigated the presence of uropathogens producing extended-spectrum beta-lactamases (ESBL) and methicillin-resistant S. aureus (MRSA) throughout the study.
| Drug | Resistant Rate, % | Sensitive Rate, % |
|---|---|---|
| Cefepime | 100 | |
| Ampicillin | 94.5 | |
| Cefazolin | 88.7 | |
| Trimethrompinsulphamethaxole | 86.6 | |
| Cefixime | 83.3 | |
| Cefuroxime | 82.7 | |
| Ceftriaxone | 82.4 | |
| Penicillin G | 80 | |
| Piperacillin | 73.7 | |
| Ciprofloxacin | 67.7 | |
| Ceftazidime | 67.1 | |
| Levofloxacin | 54.2 | |
| Amoxicillin clavulanic acid | 47.3 | |
| Tobramycin | 44.4 | |
| Erythromycin | 42.9 | |
| Gentamicin | 41.6 | |
| Cefoxitin | 38.7 | |
| Tigecycline | 100 | |
| Colimycin | 100 | |
| Vancomycin | 100 | |
| Daptomycin | 100 | |
| Linezolid | 100 | |
| Aztreonam | 100 | |
| Teicoplanin | 94.8 | |
| Fosfomycin | 94 | |
| Amikacin | 93.7 | |
| Ertapenem | 93.2 | |
| Imipenem | 91.7 | |
| Clindamycin | 89 | |
| Meropenem | 86.1 | |
| Nitrofurantoin | 81.8 | |
| Fusidic acid | 70.4 | |
| Piperacillin-tazobactam | 66.7 | |
| Tetracycline | 66.7 |
The ESBL cases constitute 10.2% of the total cases and MRSA about 0.6% of the total patients. Three of the uropathogens produced ESBL, of which E. coli was the main in 7.2%, followed by K. pneumoniae in 2.7%, and E. cloaca against 0.3% of the total ESBL cases. Ampicillin showed the highest resistance pattern against E. coli isolates in 94.8%, followed by cephazolin in about 88.4%, and trimethoprim/sulfamethoxazole against 85.1% of the samples. In contrast to these findings, tigecycline, vancomycin, linezolid, and teicoplanin demonstrated a 100% sensitivity rate among all E. coli isolates. Fluoroquinolone resistance against E. coli isolates was found in 65.3% of the cases. In contrast, fosfomycin and nitrofurantoin resistance against E. coli species was found in 3.6% and 9.4% of the cases, respectively. Table 3 demonstrates the antimicrobial resistance level against individual pathogens. Cephalosporins in 94.1%, fluoroquinolones in 82.9%, Beta-lactam in 52.2%, and aminoglycosides in 28.8% of the samples were resistant to ESBL pathogens. Although the resistance rate of carbapenems to ESBL uropathogens was 5.2%, we found that carbapenems faced increasing ESBL resistance rates (Table 4).
| Drug | Resistant Level Against Individual Pathogens, % | |||
|---|---|---|---|---|
| Escherichia coli | Klebsiella pneumonia | Pseudomonas aeruginosa | Acinetobacter baumannii | |
| Ampicillin | 94.8 | 100 | 100 | 100 |
| Amoxicillin clavulanic acid | 41.7 | 57.9 | 100 | |
| Piperacillin-tazobactam | 14.3 | 20 | 23.1 | 100 |
| Cefuroxime | 81.5 | 78.4 | ||
| Cefoxitin | 29.2 | 50 | ||
| Cefixime | 81.9 | 82.9 | ||
| Ceftazidime | 43.5 | 40 | 53.8 | 100 |
| Ceftriaxone | 80.9 | 70 | ||
| Cefazolin | 89.1 | 70 | ||
| Ciprofloxacin | 68 | 60.6 | 60 | 100 |
| Levofloxacin | 62.5 | 25 | 50 | 100 |
| Ertapenem | 6.2 | 10 | ||
| Imipenem | 3.6 | 8.1 | 21.4 | 100 |
| Meropenem | 5.7 | 0 | 25 | 100 |
| Amikacin | 4.8 | 8.1 | 14.3 | 42.9 |
| Gentamicin | 38.7 | 40 | 53.9 | 100 |
| Fosfomycin | 3.6 | 3.7 | 0 | 100 |
| Nitrofurantoin | 9.4 | 27.6 | 0 | 100 |
| Trimethrompinsulphamethaxole | 85.1 | 94.4 | 100 | 100 |
| Tigecycline | 0 | 0 | 0 | 0 |
| Colimycin | 0 | 0 | 0 | 0 |
| Vancomycin | 0 | 0 | 0 | 0 |
| Linezolid | 0 | 0 | 0 | 0 |
| Teicoplanin | 0 | 0 | 0 | 0 |
| Classes of Antibiotics | Resistant Level Against Pathogens, % | ||||
|---|---|---|---|---|---|
| Escherichia | Klebsiella | Pseudomonas | Acitinobacter | ESBL | |
| Beta lactams | 28 | 39 | 30 | 100 | 52.2 |
| Cephalosporins | 67.7 | 65.2 | 76.9 | 100 | 94.1 |
| Fluoroquinolones | 65.3 | 42.8 | 55 | 100 | 82.9 |
| Carbapenems | 5.2 | 6.3 | 15.5 | 100 | 5 |
| Aminoglycosides | 21.8 | 24.1 | 34.1 | 71.5 | 28.8 |
Multidrug-resistant microorganisms that are resistant to two or more drugs were investigated throughout the study and found in 88.7% of the cases. Acinetobacter baumannii was the most prevalent pathogen that belonged to MDR and XDR patterns in 69.1% of cases. Escherichia coli and K. pneumonia showed similar MDR patterns in 35.2% of the cases (Table 5). Some cases with prolonged hospitalization and catheterization time (about 9.4% of the total cases) had Candida species in urine cultures.
| Multidrug-Resistant Pattern | |||
|---|---|---|---|
| With MDR | Without MDR | P Value | |
| Gender | 0.008 | ||
| Female | 145 | 21 | |
| Male | 153 | 7 | |
| Patient type | 0.006 | ||
| Outpatient | 177 | 24 | |
| Inpatient | 121 | 4 | |
| Comorbidities | 0.003 | ||
| Renal failure | 79 | 2 | |
| Diabetes | 35 | 4 | |
| Stone | 33 | 2 | |
| Benign prostate hyperplasia | 26 | 1 | |
5. Discussion
Identifying the characteristic of uropathogens and antimicrobial sensitivity and resistance patterns play a crucial role to successfully treat and decide empiric treatment for the patients who are complaining of UTIs. Various studies have reported the nature and different rates of antimicrobial sensitivity and resistance patterns against uropathogens (11, 12). Our main aim in the study was to investigate the overall prevalence and pathogens and comorbidities associated with UTIs, and also antibiotic sensitivity and resistance patterns to UTIs as well as to determine appropriate empirical antibiotics to treat UTIs in community and hospitalized patients. The prevalence of uropathogens that showed growth in the urine cultures was 14.5%. We studied a total of 2,845 patients, and females displayed a slight predominance in our research (51% of the total cases), which is consistent with other studies conducted in Ethiopia, India, and Saudi Arabia (11-13). This could be due to several predisposing factors specific to women (14).
The most common prevailing uropathogen in both community- and hospital-acquired UTI in the current study was E. coli in 63.4% of the cases (two-thirds), followed by K. pneumonia (13.3%), and P. aeruginosa (3.9%), which is comparable to the previous studies (12, 13). The present study demonstrated the highest resistance rate to uropathogens by cefepime (100%), ampicillin (94.5%), cefuroxime 94.5%), cefazolin (88.7%), cefixime (83.3%), and ceftriaxone (82.4%), which is consistent with earlier studies conducted in Ethiopia and India (11, 15). One of the significant findings of our study was that fluoroquinolones (ciprofloxacin in 67.7% and levofloxacin in 54.2%) indicated increasing resistance rate to common uropathogens that is a sorrowful finding to the world that can be due to the use of fluoroquinolones as over-the-counter drugs in mild infections before the initials medications. This resistance pattern was in contrast to most of the previous studies (16, 17).
The study found the highest antimicrobial sensitivity rate (about 100%) against uropathogens in tigecycline, colimycin, vancomycin, linezolid, and teicoplanin. Moreover, fosfomycin, nitrofurantoin, and amikacin also manifested a significant sensitivity rate ranging from 86% - 95% against uropathogens (18, 19). In this study, the frequency of MDR microorganisms to two or more drugs was found in 88.7% of the cases, whereas previous studies reported a lesser rate of MDR bacterial isolates (20). This high MDR in the present study might be due to improper prescription of antibiotics, epidemic misuse of antimicrobials, self-prescription of antibiotics, and the lack of knowledge about drug resistance in our country. Acinetobacter baumannii was the most prevalent pathogen that belonged to MDR and XDR patterns in 69.1% of cases. E. coli and K. pneumonia showed similar MDR patterns in 35.2% of the cases.
Another significant finding of our study was that trimethoprim/sulfamethoxazole (85.1%) and ciprofloxacin (68%) against E. coli exceeded the recommended local resistance level for empirical therapy (about < 20% and < 10%, respectively) (21)). Acinetobacter baumannii was the most resistant uropathogen in our study and showed 100% resistance rates against beta-lactam, cephalosporins, fluoroquinolones, and carbapenems. Significant morbidity, mortality, prolonged hospitalization, need for intensive care unit admissions, and increased health care costs were found in our patients. We investigated the presence of ESBL-producing uropathogens throughout the study that included 10.2% of the total cases. Overall, E. coli species accounted for about 1.1% of ESBL production, whereas K. pneumonia accounted for about 20.8%. Klebsiella pneumonia had higher antimicrobial resistance compared with E. coli regarding ESBL production. High antimicrobial resistance to cephalosporins (94.1%) and fluoroquinolones (82.9%) in ESBL-producing isolates was noted in our study and has also been reported in previous studies.
In all urine cultures, P. aeruginosa showed growth in 3.9% of the cases, and Pseudomonas was 100% resistant to trimethoprim/sulfamethoxazole, ampicillin, and cefepime. Also, was found 21-53.8% resistance rate against piperacillin-tazobactam, amikacin, imipenem, and ceftazidime that is in contrast to a study conducted in India by Manjunath et al. (15). Interestingly, fosfomycin and nitrofurantoin showed a 0% resistance rate against Pseudomonas. Pseudomonas aeruginosa revealed a 29.5% MDR pattern. Cedecea lapagei is a very rare pathogen of UTIs and is extensively resistant to the ESBL antibiotics and ESBL inhibitors that was found in our study for the first time. Fortunately, carbapenems, fluoroquinolones, aminoglycosides, and trimethoprim-sulfamethoxazole showed a higher sensitivity rate against pathogens. Although the pattern and distribution of uropathogens are different in countries, a continuous assessment for the changing trends of antimicrobial sensitivity and resistance toward uropathogens is indispensable.
5.1. Conclusions
We reported increased trends of antimicrobial resistance in trimethoprim/sulfamethoxazole (85.1%) and fluoroquinolones (61%) against E. coli that was higher than the recommended local resistance level for empirical therapy (< 20% and < 10%, respectively). We also suggest using fosfomycin, and nitrofurantoin for UTI empiric treatment, and other antibiotics should be prescribed carefully. Changes in the pattern of antimicrobial resistance are related to increased morbidity, unnecessary hospital admissions, prolonged hospital stay, and high healthcare costs.