1. Background
Leishmaniasis is an infectious disease in tropical areas and encompasses several types, including (i) cutaneous leishmaniasis (CL) with benign cutaneous scars, (ii) mucocutaneous leishmaniasis with chronic ulcerative lesions, and (iii) visceral leishmaniasis, i.e. an acute visceral form. Cutaneous leishmaniasis is induced by Leishmania major and L. tropica worldwide, especially in Iran (1). Leishmania parasites make intracellular protozoa infect reticuloendothelial macrophages in the vertebrate hosts as amastigote form while as a motile flagellated promastigote invertebrate sand fly vector (1, 2). Dry-type CL, induced by L. tropica, is reported just in eight different cities, while wet-type CL is observed in many regions of the country with a 95% prevalence rate (3, 4). A standard CL treatment is antimony-containing agents such as meglumine antimonite or glucantime (Glu) and sodium stibogluconate (Pentostam) with intralesional or systematic administration; however, some studies have reported resistance to these agents (5-8). Accordingly, the development of new anti-leishmanial therapeutic agents, especially combination therapy, seems necessary.
Artemisinin (Art) is a sesquiterpene produced by Artemisia annua L. and is currently the best anti-malaria agent (9-11). Artemisinin has also revealed in vitro and in vivo anti-leishmanial effects (12, 13); however, the immune system plays an critical role in the initiation and development of leishmaniasis (14). When T-cells’ activity is interrupted, and macrophages fail to start the phagocytosis of Leishmania parasites, the disease would emerge and develop. Th1 and Th2 cells are the main immune system components against leishmaniasis, which produce interferon-γ (INF- γ) and interleukin-4 (IL-4), respectively. Accordingly, the modulation of immune systems is of essence for the treatment of leishmaniasis (15).
Studies have shown that Art plays a dual role in leishmaniasis control. It (i) increases the production of NO and iNOS in uninfected macrophages and (ii) indirectly modulates the immune system by increasing the expression of Th1 cytokines (16). Shark cartilage extract (ShCE) has also revealed some immunomodulatory effects (17). It has been used in the treatment of several diseases: angiogenesis inhibitor in the treatment of cancer (18), lubricant in the treatment of arthritis (19), and the treatment of psoriasis and diabetic retinopathy (20). It also induces inflammatory cytokines by 14 and 15 kDa low molecular weight proteins (21).
2. Objectives
The research incentives aroused from the increasing problems of leishmaniasis in the central districts of Iran. The present study aimed to monitor the effectiveness of control measures, to modify incrimination of Leishmania parasites at their species level with molecular analyses, characterize the increased therapeutic problems of wet-type CL in Iran, and detect the effects of Art on Leishmania parasites and ShCE on immune system modulation to study the effects of these agents, either alone or in combination with Glu, on L. major in vitro and in vivo.
3. Methods
3.1. Culture of Leishmania major
Leishmania major (MRHO/IR/75/ER) was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 100 u/mL penicillin, 100 µg/mL streptomycin, and 20% fetal bovine serum (FBS). The cultures were incubated at 25 ± 1°C.
3.2. Art Preparation
Art (C15H22O5) was purchased from Holly pharmaceuticals (US), and its stock solution was prepared in 1:1 ethanol and distilled water. Eight 2-fold serial dilutions of the Art stock were prepared for the experiments (400 - 3.12 µg/mL). In the case of using the combination of Art, a similar concentration of each drug was mixed (i.e., 400 µg/mL Art + 400 µg/mL Glu, 200 µg/mL Art+ 200 µg/mL Glu, etc.). For the in vivo experiments, 25 µg/mL concentration of Art was used to prepare ointment with a vaseline base (250 µg Art in 10 mL Vaseline) (22).
3.3. Glu Preparation
Glu (Sanofi-Aventis, France) was prepared for the experiments at 3.12-400 µg/mL concentration, as described in the Art Section (23).
3.4. Preparation of ShCE
Neural cord cartilage was prepared from a Dogfish shark (Persian Gulf, Iran) (21, 23). Four hundred µg/mL of ShCE was used for all the concentrations of assays in vitro. Moreover, 0.5 mL/mouse (20 mg/kg) was used in mice orally daily (24).
3.5. Promastigote Inhibition
To determine the IC50 (half-maximal inhibitory concentration) of the drugs, L. major promastigotes were exposed to different concentrations of Art, Glu, Art-Glu, and ShCE, as described in our previous studies on L. infantum (23-25). IC50 was calculated based on the promastigote counts.
3.6. MTT Assay for Promastigote Viability
The MTT assay was used to evaluate the cytotoxic effects of the drugs and their combinations. Briefly, 106/mL of log-phase promastigotes was exposed to different concentrations of Art, Glu, Art-Glu, Art-ShCE, Glu-ShCE, and ShCE. After 72h of incubation at 24°C, MTT solution (20µL) was added to each well and then incubated at 37°C for 5h in a dark room. The plates were centrifuged at 300 rpm for 10 min, and 100 µL Dimethyl sulfoxide (DMSO) was added to the pellets (26). After 10 min, the optical densities (OD) were measured at 570nm, according to which the viability percentage was estimated as follows: Viability percentage = (Absorbance of treated cells/Absorbance of control cells) ×100
3.7. Amastigote Assay
3.7.1. Macrophage Culture and Infected Macrophage Preparation
The J774 macrophage cells were cultured in RPMI 1640 supplemented with 10% FBS, penicillin G (100 IU/mL), and streptomycin (100µg/mL) and then incubated at 37°C with 5% CO2. To prepare the infected macrophages, the stationary phase of L. major promastigotes was added to the cultured macrophages (10 parasites/ macrophage) and then incubated at 37°C with 5% CO2 and 95% humidity for 24 h. Finally, the cultures were washed to remove free parasites and re-incubated for 24 h.
3.7.2. MTT Assay for Macrophage Viability
When the macrophage culture was prepared, their culture medium was replaced with a fresh medium containing the drugs at different concentrations (400-3.12 µg/mL). After 72h, the viability of the macrophages was assessed using an MTT assay.
3.8. Anti-amastigote Experiment
The J774 macrophages (105 cells /mL) were cultured in 24-well plates and incubated for 24h. Round coverslips were also placed in the bottom of the wells. The non-adherent cells were washed by fresh RPMI and then removed. The adherent macrophages were infected by stationary-phase promastigotes at a parasite/macrophage ratio of 10:1, and then the plate was incubated with 5% CO2 at 37°C. After six hours, free promastigotes were removed by being washed with fresh RPMI. Then, 100µg/mL of drugs were added to the infected macrophages. The infected macrophages with no drug were used as a negative control. The macrophages were then fixed in methanol and stained with Giemsa. The percentage of the infected cells and the number of amastigotes/infected cells were calculated. The results were expressed as the mean of three independent tests. Promastigote and amastigote assay experiments were performed in triplicate.
3.9. In Vivo Experiments
One hundred and forty 5 - 7-week-old female inbred BALB/c mice (mean weight: 16 - 18 g) were purchased from the Pasteur Institute of Iran. The mice were transferred to the Animal House of the Tarbiat Modares University and kept at 22 ± 1°C in a 12-h day/night cycle. They were provided with a standard pellet diet and water ad libitum. The mice were then assigned into 20 groups, with seven mice per group. They were treated using either the drugs alone or the combination of the drugs with different routes of administration, as presented in Table 1. To prepare the L. major model of leishmaniasis, the mice were inoculated in the tail with 107 stationary-phase promastigotes. The treatments started 14 consecutive days following the emergence of the disease. The doses of the drugs during treatment of the mice were as follows: (i) 25 µg/mL of Art orally, intraperitoneally, or as an ointment; (ii) 20 mg/kg/ bodyweight of Glu (85mg/mL of antimony) intraperitoneally, intralesionally, intramuscularly, and subcutaneously; (iii) 0.5 mg/kg/bodyweight of oral ShCE (
| Mice Groups | Route of Administration | Abbreviation | |
|---|---|---|---|
| 1 | Uninfected-untreated | - | CTRL (-) |
| 2 | Infected-untreated | - | CTRL (+) |
| 3 | Artemisinin-treated | Oral | Artoral |
| 4 | IP | ArtIP | |
| 5 | Oint | Artoint | |
| 6 | Glucantime-treated | IP | GluIP |
| 7 | IL | GluIL | |
| 8 | IM | GluIM | |
| 9 | SC | GluSC | |
| 10 | ShCE treated | Oral | ShCE |
| 11 | Artemisinin oral- Glucantime Intraperitoneal treated | Oral-IP | ArtoralGluIP |
| 12 | Artemisinin oral- Glucantime Intramuscular treated | Oral-IM | ArtoralGluIM |
| 13 | Artemisinin ointment- Glucantime Intraperitoneal treated | Oint-IP | ArtointGluIP |
| 14 | Artemisinin ointment- Glucantime Intramuscular treated | Oint-IM | ArtointGluIM |
| 15 | Artemisinin oral- Shark Cartilage Extract treated | Oral | ArtoralShCE |
| 16 | Artemisinin ointment- Shark Cartilage Extract treated | Oint-Oral | ArtOintShCE |
| 17 | Glucantime Intraperitoneal- Shark Cartilage Extract treated | IP-Oral | GluIPShCE |
| 18 | Glucantime Intramuscular- Shark Cartilage Extract treated | IM-Oral | GluIMShCE |
| 19 | Ethanol (As Artemisinin solvent) treated | IP | Ethanol |
| 20 | Vaseline- treated (Ointment base) | Oint | Vaseline |
Abbreviations: IP, intraperitoneal; IM, intramuscular; IL, intralesional; SC, subcutaneous; Oint, ointment.
3.10. Parasite Burden and Real-time PCR (RT-qPCR)
The number of parasites in the spleen and lesions of mice (5 mice of each group were sacrificed) was determined using the RT-qPCR assay in the fourth post-treatment week (50 days after the inoculation of parasites). RNA was extracted from spleen and liver tissues (30 mg) using RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Then cDNA was synthesized using Quanti Tect Reverse Transcription Kit (Qiagen). About 107 promastigotes were harvested and washed twice with Phosphate-buffered saline (PBS), and RNA was extracted to generate a standard curve. The RNA concentration and purity were evaluated using A260 spectrophotometry. The primers were designed for the kDNA gene of L. major using NCBI primer design tool.
The primers’ sequences were as follows: forward: 5´-GCGGGTACCATGCAGGGGACTTGGTTTTC-3´ and reverse: 5´-CGGGGAATTCTCACTCTTTGCGGATTCTTT-3´.
RT-qPCR was performed by using the Light Cycler system and Taq DNA Polymerase 2X Master Mix RED (Viragen Diagnostic) as well as 2 μL (10 pg) of the cDNA template. The PCR protocol was as follows: initial denaturation at 95°C for 10 min followed by 45 cycles of 95°C for 10 s, 54°C for 10 s, and 72°C for 25 s. The final extension step at 72°C for 10 min was finally added. The test was perfomed in triplicates. First, mean threshold cycles (Cq) and standard deviation (SD) were calculated, and then a standard curve of mean Cq against parasite number was prepared (24).
3.11. Lesion Sizes
The size of the lesions was monitored using a caliper per six days. The measurements started before the treatment (week 0) and during the treatment (weeks 1, 2, 3, and 4).
3.12. Cytokine Assay
Five mice from each group were sacrificed, and spleen lymphocytes were extracted to measure INF-γ and IL-4 levels, as described in the previous studies (24, 25).
3.13. Statistical Analysis
Differences among the groups were determined using One-Way ANOVA and Tukey-Krammer tests. T-test was used to compare the groups in pairs. P < 0.05 was set as the significance level. SigmaPlot 12.3 was used to determine IC50 values.
4. Results
4.1. In vitro Experiments Promastigote Assay Inhibition Test
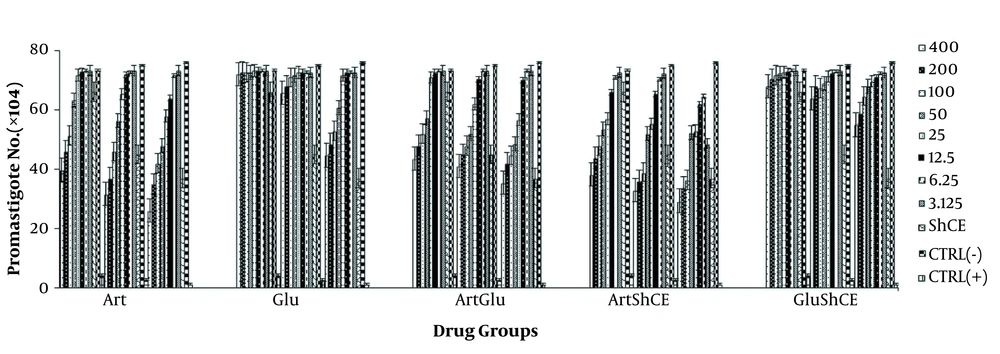
There were fewer parasites in the Art group after the administration of all Art concentrations, except for 3.12 µg/mL and 6.2µg/mL after 24 h. All Art concentrations, except for 3.12µg/mL after 48 h, reduced the number of parasite. The parasites were fewer than the control group at all Art concentration after 72 h, and Glu had effects on promastigotes after 48 h (P < 0.05). Regarding the drug combinations, the greatest synergistic effect was observed in Art-ShCE and Art-Glu groups after 72 h, respectively. Moreover, ShCE had a significant effect on promastigotes at all time points (Figure 1). The IC50 of Art, Glu, Art-Glu, Art-ShCE, and Glu-ShCE on promastigotes after 72h were 29.05 ± 1.53, 279 ± 2.18, 34.1 ± 2.2, 21.8 ± 1.9, and 329 ± 1.59 respectively (Figure 1).
Number (×104) of promastigotes treated by different concentrations of drugs and control group after 24, 48, and 72h (n = 3). Values are expressed as mean ± SD. ANOVA analysis reveals the statistical differences between the groups (P = 0.001). The greatest synergistic effect was observed for Art-ShCE and Art-Glu.
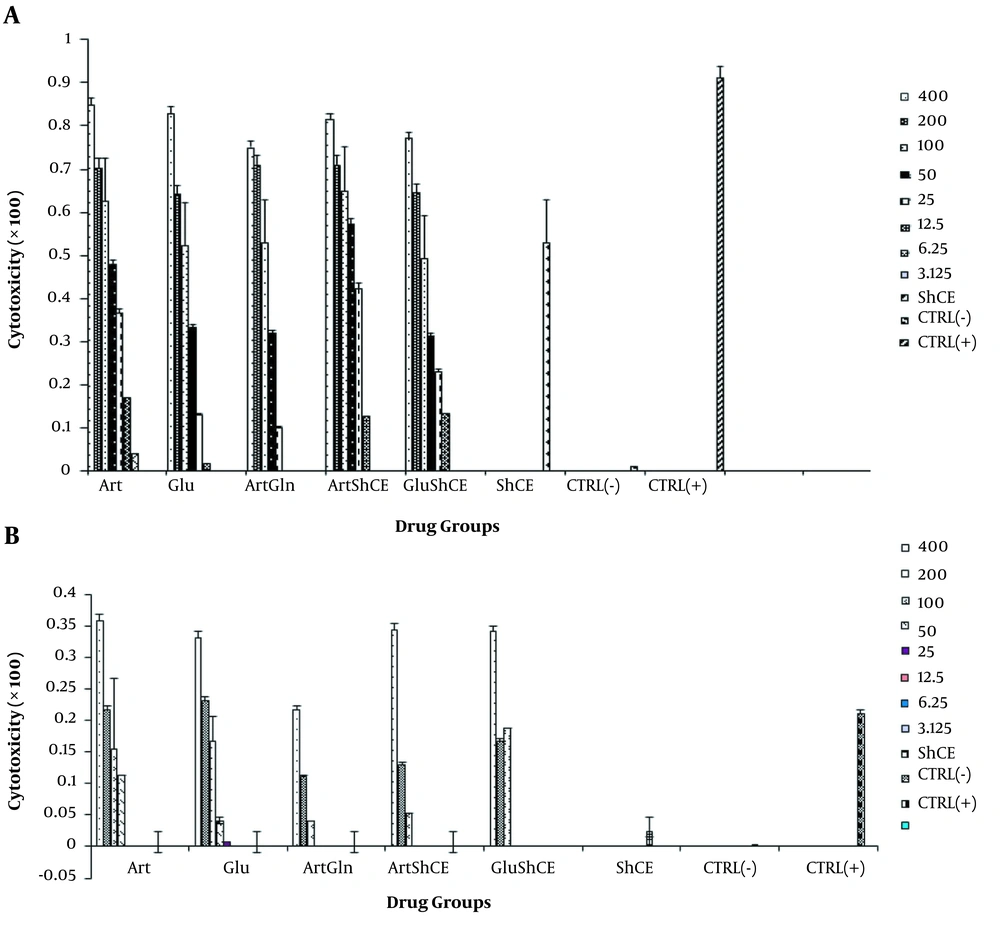
4.2. MTT Assay
The cytotoxic effects of the drugs on promastigotes and uninfected macrophages are presented in Figure 2A and B. As it can be noticed, ShCE, Art-ShCE, and Art-Glu have high cytotoxic effects. Note that the cytotoxic effect of Art on promastigotes was greater than that of Glu (P < 0.001). On the other hand, ShCE had a small cytotoxic effect on uninfected macrophages, and Glu was more cytotoxic than Art for uninfected macrophages (P < 0.05) (Figure 2).
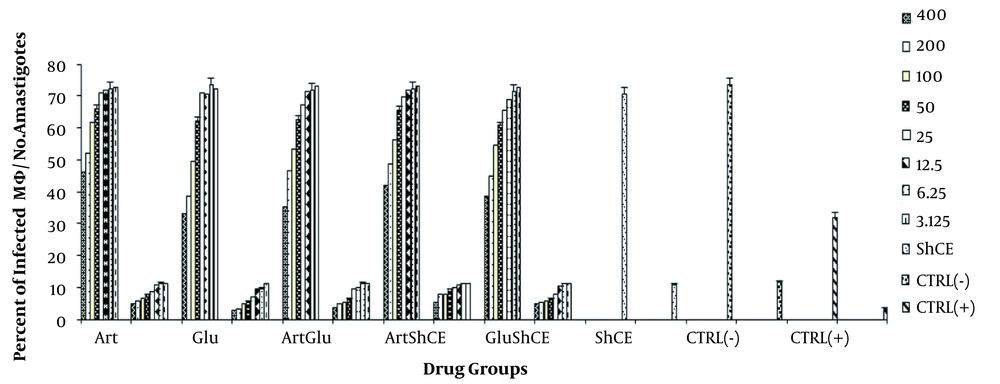
4.3. Amastigote Assay
In this study, the effects of the drugs on infected macrophages and amastigotes were greater than that of the promastigotes (P < 0.05). This was more remarkable for Glu. In general, the effect of Glu on amastigotes was greater than that of promastigotes. Unlike promastigotes, the effects of ShCE on infected macrophages and amastigotes were negligible. Regarding the drug combinations, the effects of Art-Glu on amastigotes were greater than other combinations; however, the difference was not statistically significant (P > 0.05) (Figure 3). The IC50 of Art, Glu, Art-Glu, Art-ShCE, and Glu-ShCE on amastigotes after 72h were 83.7 ± 0.73, 59.5 ± 0.57, 54.1 ± 0.22, 89.8 ± 0.9, and 51.2 ± 0.39, respectively.
4.4. In Vivo Experiments
4.4.1. Parasite Burden
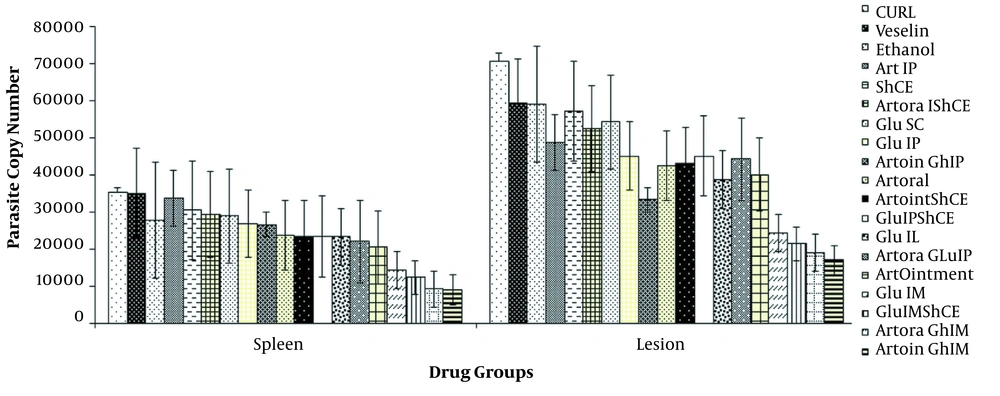
Figure 4 presents the RT-qPCR assay results on the spleen and the lesions. The parasite burden was lower on the spleen than on the lesions after the treatment (P < 0.05). The intramuscular administration of Glu and Art ointment resulted in minimum parasite burden. Additionally, oral administration was better than IP administration. The intra-lesional injection of Glu provided better results than the IP administration; however, the intra-lesional administration resulted in a bad look at the lesions. The drug combinations resulted in a lower parasite burden than the single drugs. As shown in Figure 4, the IM administration of Glu-Art ointment and then the IM administration of Glu and oral Art had the highest effect on the parasite burden.
The Glu-ShCE was more effective than the Art-ShCE combination (P < 0.05). Although ShCE and oral Art groups revealed a statistically significant difference with the control group, no effective result was observed. The results of the parasite burden on the lesions of all the treatment groups were statistically significant, compared to the control group (P < 0.05). On the other hand, the parasite burden on the spleen of the Art-IP (P = 0.9), ShCE (P = 0.4), and Vaseline (P = 0.1) groups was not significantly different from that of the control group. The other groups, however, were statistically and significantly different from the control group (P < 0.05).
4.2. Lesion Size
The lesion size increased in the control and Vaseline groups at the end of the fifth weeks after the emergence of the lesions. In the other groups, the size of the lesions decreased in the first week after the treatment. Then the size of the lesion increased during the second week. Afterwards, the size of the lesions decreased until the end of the fifth week after the treatment. The efficacy of the drug combinations was ArtointGluIM< GluIMShCE< Glu IM<ArtoralGluIM< ArtOint< ArtoralGLuIP, with Artoint-GluIM and ArtoralGLuIP having the highest and lowest efficacy, respectively. Ethanol was used as Art solvent and significantly affected the lesion size reduction (Table 2).
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|---|
| CTRL | 3.83 ± 0.2 | 6.2 ± 0.17 | 8.73 ± 0.20 | 12.86 ± 0.16 | 14 ± 0.31 | 15.56 ± 0.28 |
| Vaseline | 4.3 ± 0.2 | 7.73 ± 0.33 | 8.76 ± 0.56 | 10.9 ± 0.17 (P < 0.05) | 12.8 ± 0.15 | 14.43 ± 0.12 (P < 0.05) |
| Ethanol | 5.033 ± 0.14 | 7.06 ± 0.1 | 8.76 ± 0.5 | 5.93 ± 0.74 (P < 0.05) | 3.23 ± 0.29 (P < 0.05) | 2.6 ± 0.12 (P < 0.05) |
| Art IP | 6.16 ± 0.33 | 7.2 ± 0.4 | 7.66 ± 0.16 | 6.46 ± 0.39 (P < 0.05) | 6.3 ± 0.14 (P < 0.05) | 6.26 ± 0.13 (P < 0.05) |
| ShCE | 5.9 ± 0.37 | 6.73 ± 0.61 | 7.66 ± 0.13 | 5.8 ± 0.49 (P < 0.05) | 5.83 ± 044 (P < 0.05) | 6.23 ± 0.14 (P < 0.05) |
| ArtoralShCE | 4.76 ± 0.14 | 6.66 ± 0.16 | 7.46 ± 0.26 (P < 0.05) | 5.43 ± 0.52 (P < 0.05) | 5.03 ± 0.26 (P < 0.05) | 5.16 ± 0.16 (P < 0.05) |
| Glu SC | 5.83 ± 0.44 (P < 0.05) | 6.76 ± 0.14 | 7.46 ± 0.26 | 5.73 ± 0.5 (P < 0.05) | 4.93 ± 0.16 (P < 0.05) | 4.93 ± 0.29 (P < 0.05) |
| Glu IP | 4.76 ± 0.62 | 6.46 ± 0.31 | 8.36 ± 0.22 | 5.5 ± 0.29 (P < 0.05) | 4.16 ± 0.29 (P < 0.05) | 4.16 ± 0.16 (P < 0.05) |
| ArtointGluIP | 5.66 ± 0.28 (P < 0.05) | 6.16 ± 027 | 6.96 ± 0.03 | 4.7 ± 0.36 (P < 0.05) | 4.53 ± 0.40 (P <0.05) | 4.23 ± 0.12 (P < 0.05) |
| Artoral | 5.73 ± 0.62 (P < 0.05) | 6.23 ± 0.31 | 7.26 ± 29 | 5.9 ± 0.28 (P < 0.05) | 6.033333 ± 0.16 (P < 0.05) | 5.4 ± 0.29 (P < 0.05) |
| ArtointShCE | 5.43 ± 0.56 (P < 0.05) | 6.76 ± 0.14 | 7.56 ± 0.17 | 5.43 ± 0.52 (P < 0.05) | 4.46 ± 0.08 (P < 0.05) | 4.03 ± 0.08 (P < 0.05) |
| GluIPShCE | 6.1 ± 0.1 (P < 0.05) | 7 ± 0.28 | 7.8 ± 0.15 | 5.43 ± 0.52 (P < 0.05) | 5.06 ± 0.14 (P < 0.05) | 5.23 ± 0.16 (P < 0.05) |
| Glu IL | 5.83 ± 0.20 | 6.4 ± 0.38 | 6.93 ± 0.23 (P < 0.05) | 6.4 ± 1.05 (P < 0.05) | 4.66 ± 0.36 (P < 0.05) | 4.66 ± 04 (P < 0.05) |
| ArtoralGLuIP | 5.5 ± 035 (P < 0.05) | 5.9 ± 0.37 | 6.6 ± 0.37 (P < 0.05) | 5.56 ± 0.21 (P < 0.05) | 3.9 ± 0.3 (P < 0.05) | 3.4 ± 0.1 (P < 0.05) |
| ArtOintment | 5.53 ± 0.81 (P < 0.05) | 5.83 ± 0.44 | 6.7 ± 0.47 (P < 0.05) | 5.6 ± 0.15 (P < 0.05) | 3.26 ± 0.33 (P < 0.05) | 3.16 ± 0.29 (P < 0.05) |
| Glu IM | 5.23 ± 0.43 (P < 0.05) | 5.1 ± 0.2 | 4.73 ± 0.37 (P < 0.05) | 3.36 ± 0.27 (P < 0.05) | 2.03 ± 0.04 (P < 0.05) | 2.03 ± 0.03 (P < 0.05) |
| GluIMShCE | 5.83 ± 0.8 | 5.83 ± 0.32 | 4.1 ± 0.2 (P < 0.05) | 3.66 ± 0.14 (P < 0.05) | 2.13 ± 0.21 (P < 0.05) | 2.1 ± 0.39 (P < 0.05) |
| ArtoraGluIM | 4.43 ± 0.23 | 4.9 ± 0.2 (P < 0.05) | 7.53 ± (P < 0.05) | 4.43 ± 0.43 (P < 0.05) | 3.73 ± 0.21 (P < 0.05) | 2.63 ± 0 .17 (P < 0.05) |
| ArtoinGluIM | 5.5 ± 0.28 | 4.53 ± 0.14 | 6.46 ± 0.48 | 4.93 ± 0.22 (P < 0.05) | 2.53 ± 0.88 (P < 0.05) | 1.76 ± 0.18 (P < 0.05) |
aP-values greater than 0.05 are not shown.
4.3. Cytokine Assay
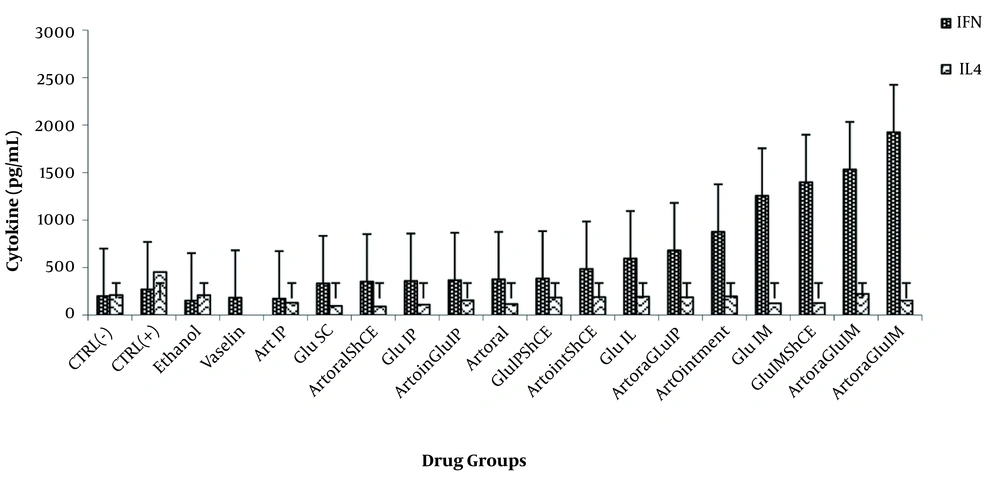
INF-γ and IL-4 levels were evaluated in all the groups (Figure 5). The level of IFN-γ was higher in all the groups than in the positive and negative control groups (P < 0.05). Moreover, the IL-4 level was higher in control infected mice than in the treatment groups (P < 0.05). There was a statistically significant difference between the IL-4 levels in all the groups compared to the positive control group (P < 0.05). However, the IFN-γ levels in the GluIL, GluIM, ArtOintment, ShCE, ArtoralGluIP, ArtOralGluIM, ArtintGluIM, ArtOintShCE, GluIMShCE groups had a statistically significant difference with the positive control groups (P < 0.05).
5. Discussion
The present study revealed that Art and Glu alone had inhibitory effects on promastigotes. Art-ShCE and then Art-Glu combinations also had the best synergistic effects on promastigote inhibition. Moreover, ShCE, ShCE-Art, and ShCE-Glu had high cytotoxic effects on promastigotes and low cytotoxic effects on uninfected macrophages. All the drugs had greater effects on amastigotes and infected macrophages than promastigotes. In this regard, Art-Glu had the highest effect on amastigotes. Parasite burden was also reduced on the spleens and lesions of the treated mice. Moreover, the treatment increased INF-γ and decreased IL-4 and lesion size. According to Cortes et al., Art has inhibitory effects on promastigotes and amastigotes of Leishmania ranging between 3.51µM and 1.25mM for promastigotes and between 79.76µM and 1.20 mM for amastigotes (27).
Ghaffarifar et al. also showed that 100µg/mL of Art could inhibit 81% of L. major promastigotes (22). In Esavand Heydari’s study, Art had an IC50 of 50 µg/mL on L. major. In the present experiment, the IC50 of Art was 29.05 ± 1.53µg/mL, which is higher than those reported by Cortes and Heydari. Esavand Heydari et al. explored the toxic effects of Art and Artemisinin sieberi on promastigotes and mouse macrophages (28). They found that although Artemisia sieberi was more effective than Art, Art was considerably cytotoxic for promastigotes. Art could reduce promastigote viability by 40% after 72 h. Furthermore, the cytotoxic effect of Art on uninfected macrophages was negligible. In another study by Sen et al., the researchers showed that Art reduced the viability of Leishmania sp. promastigotes by about 25%.
Sen et al. showed that Art was toxic to uninfected macrophages and induced the production of macrophage nitric oxide as an essential element in eliminating Leishmania parasites (29). The anti-leishmanial effects of Art are associated with the cleavage of its endoperoxidase bridge, which results in the production of oxygen radicals. Given the relatively compromised anti-oxidant system of the Leishmania parasite, oxygen radicals can readily inhibit Leishmania mitochondrial complexes (30). On the other hand, Glu exerts its anti-leishmanial effects by oxidative stress-derived DNA damage and is equally detrimental to human and Leishmania cells (31). In this study, although Glu had inhibitory effects on promastigotes, Art was more effective than Glu in all doses at all time points. Furthermore, Glu was less toxic than Art for promastigotes and had higher toxic effects than Art on macrophages. In contrast, the researcher in a study on 49 patients reported the patients’s complain about myalgia (32). Other studies have also reported cardiotoxicity and skin reaction because of the heavy metals existing in antimoniate-based therapeutics (33, 34).
To the best of our knowledge, no study, except for our previous two studies, has examined the effects of ShCE on Leishmania promastigotes (23, 24). Our findings revealed that ShCE had an inhibitory effect on promastigotes at all time points; however, the mechanism of action of shark cartilage is unclear. The most potent protein fraction stimulating the immune response or even the anti-promastigote effect of ShCE is associated with low-molecular-weight proteins, as used in this study (14 - 15 kDa) (21). The effects of the drug combinations on promastigotes also showed that Art combined with either ShCE or Glu had a promastigote inhibitory effect as such Art-ShCE was more effective than Glu-ShCE. Although Art-ShCE was more effective than Art-GLu, the diffrence was not statistically significant.
Interestingly, the Art-ShCE cytotoxic had smaller effects on uninfected macrophages than GLu-ShCE and Art-Glu, indicating the synergistic effect of Art and ShCE on promastigote inhibition and the low toxicity of Art-ShCE on uninfected mouse macrophages. The findings of the present study also revealed that all the drugs and their combinations, especially Glu, had greater effects on amastigotes and infected macrophages than promastigotes. The results showed that the combinations are more effective than the single drugs. Art-Glu was more effective than the other combinations; however, the difference was not statistically significant. These findings are in line with those in other studies. For example, Musfikur et al. showed that the combination of art-amphotericin B and art-miltefosine is more effective than either of the drugs (35).
All the drugs and their combinations reduced the parasite burden on the spleen and lesions, compared to the control groups. The in vivo experiments showed that the IM administration of Glu-Art ointment and then the IM administration of Glu-ShCE caused the least parasite burden on the spleen, compared to single drugs and other drug combinations. All the drugs and their combinations in the lesions resulted in a lower parasite burden, compared to the control groups. Lesion size was smaller in all the treatment groups than in the control groups. Note that the most effective treatment was ArtoralGLuIP.
According to the cytokine analysis, INF-γ and IL-4 levels were higher and lower in the treatment and control groups, respectively. However, the parasite burden was lower on the spleen than on the lesions. A review of the literature indicates that Art is highly effective in decreasing parasite burden (28, 29), which seems to be associated with the direct anti-leishmanial effects of Art rather than nitric oxide-dependent pathways (29). Mostafavi et al. indicated that Glu effectively reduced parasite burden more than four folds in BALB/c mice compare to control groups (36). In sum, the present findings suggest that the best route of administration is Artoint-GluIM, followed by Artoral-GluIM.
Lesion size was also reduced five weeks after the treatment in the treatment groups but not in the control groups. The most effective combinations were ArtoralGLuIP, Artointment, ArtoraGluIM, Glu IM, GluIMShCE, and ArtoinGluIM. The direct contact and penetration of the therapeutic drugs into the lesions may justidy the high efficacy of the ointment (28). No study has yet documented why the IM administration of Glu is more effective than the IV administration in mice. Further studies are recommended to address this issue. In this study, ArtoralGluIM and ArtoinGluIM were also among the most effective combinations for parasite burden reduction. Aghai et al. also showed that Glu effectively reduced lesion size eight weeks after the treatment (37), and that Glu in combination with olive oil was more effective. This is in agreement with our findings, suggesting that Glu combined with herbal compounds is more effective than Glu alone.
According to the present findings, lesions size was smaller in the Ethanol (As Artemisinin solvent) treated mice than the Art treated mice at the end of the fifth weeks. This is probably related to the limited size and the dry appearance of the lesion. However, according to the parasite load results, the parasite load in the former group was more than the latter groups. The immune-modulatory effects of the anti-leishmanial agents are significant since one of the challenges in Leishmania treatment is impaired immune responses. The present study showed that the drugs and their combination resulted in an elevated INF-γ level and a declined IL-4 level in the treatment groups, compared to the positive control group. Furthermore, IL-4 was lower in all the treatment groups than the positive control group, and INF-γ was higher in the GluIL, GluIM, Artoint, ShCE, ArtoralGluIP, ArtOralGluIM, ArtOIntmentGluIM, ArtointmentShCE, and GluIMShCE groups than the positive control group. Sen et al. also claimed that Art induced the production of Th1 cytokines such as INF-γ. However, they observed no variation in the IL-4 levels after treatment with Art (29). Mostafavi et al. also evaluated the levels of Th1 and Th2 cytokines after treatment with Glu. Although they measured IL-10 and IL-12, they reached the findings similar to the present research findings, indicating that Glu increased Th1 cytokines and decreased Th2 cytokines, compared to the untreated control groups (36).
5.1. Conclusions
The findings indicate the anti-leishmanial effect of the concerned drugs. It is also revealed that the Art-ShCE combination has a more inhibitory effect on L. major promastigotes and amastigotes than other drug combinations, and that it is not toxic for uninfected macrophages. The findings also document that the best combination of Glu administration routes is IM, and that the ointment is the best Art administration route. However, further studies on animal and clinical trials are recommended to evaluate these combinations in human subjects.