1. Background
In recent years, antibiotic resistance has been the subject of much research worldwide because it has become a serious threat to the health of patients with chronic diseases, such as hemodialysis patients. Carbapenemase-producing and extended-spectrum beta-lactamase-producing Enterobacteriaceae (CPE and ESBL-E, respectively) are the leading causes of infections such as urinary tract infection and sepsis, playing a role in many therapeutic problems (1, 2). The gut is an important place for accumulating these pathogenic bacteria (3, 4). The association between bacterial colonization of the intestine by resistant bacteria and bacterial infections has been well documented in hemodialysis patients (5-7). Extended-spectrum beta-lactamase enzymes were first identified in Germany and have spread rapidly among bacteria with considerable variability. These enzymes are produced by a specific group of bacteria and can hydrolyze cephalosporins of a wide range of antibiotics such as ceftazidime, ceftriaxone, and cefotaxime. In addition, they are inhibited by clavulanic acid and tazobactam.
Carbapenemase-producing Enterobacteriaceae infections are often associated with high mortality. One of the most important reasons is the limited treatment options. The producers of Metallo beta-lactamase and OXA-48 are usually resistant to carbapenems and other beta-lactams, except for aztreonam. These resistances have caused a great deal of concern in therapeutic settings, and fecal carriers of these strains can be a serious alarm to increase the rate of urinary tract infections. The rate of bacterial colonization of the intestine is high in hemodialysis patients, predominantly Escherichia coli and Klebsiella pneumoniae (8-10). These asymptomatic carriers could spread ESBL and carbapenemase resistance genes in the community (11). These genes are often found on mobile genetic elements alongside other resistance genes and could be easily transferred to different strains (12). In the last decade, carbapenemase- and ESBL-producing Enterobacteriaceae were endemic in the Middle East (13-15). Diabetes mellitus, antibiotic consumption, and gender have been identified as the risk factors of rectal colonization with ESBL-producing bacteria (7).
2. Objectives
Hemodialysis patients who visit hospitals every day are in constant contact with fistulas, catheters, other colonized patients, and infected staff hands. They are at high risk of infection and can easily transmit resistant isolates to the community. However, such an issue has not yet been studied in Iran. Therefore, this study assessed the prevalence of fecal carriage among hemodialysis patients and the factors affecting its occurrence in a hospital in Tehran.
3. Methods
3.1. Study Population
This study was performed on 150 hemodialysis patients (98 males and 52 females) referred to a hospital in Tehran from February 2018 to April 2020. Due to kidney problems, such patients are referred to the hospital for dialysis every day. The required information was collected through a questionnaire completed by the patients to assess the risk factors of intestinal colonization, including age, sex, occupation, education, diabetes history, gastrointestinal status, antibiotic consumption, and the number of dialysis sessions.
3.2. Sample Collection and Screening
Stool samples were collected from 150 hemodialysis patients from February 2018 to April 2019 in a hospital in Tehran. Fecal specimens underwent the necessary tests within four hours of sampling. For screening, 0.5 g of each fecal sample was dissolved in 1 mL of 0.9% saline and then vortexed. This suspension was cultured on MacConkey agar. Then, four antibiotic discs containing cefotaxime (CTX, 30 µg), ceftazidime (CAZ, 30 µg), imipenem (IMI, 10 µg), and meropenem (MEM, 10 µg) were placed on the plate. Colonies were examined after 24 hours of incubation at 37°C. The colonies within the growth inhibition zone around each antibiotic disc were isolated as the strains suspected resistant to cephalosporins and carbapenems and maintained for further investigation. Furthermore, conventional biochemical tests were carried out to identify possible strains of Enterobacteriaceae.
3.3. Antibiotic Susceptibility Testing and Phenotypic Detection of ESBL
Antibiotic susceptibility testing was performed by the disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (16). The following antimicrobial agents were examined: amoxicillin/clavulanic acid (20/10 µg), cefotaxime (30 µg), ceftazidime (30 µg), ciprofloxacin (5 µg), fosfomycin (200 µg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), gentamicin (10 µg), tetracycline (30 µg), aztreonam (30 µg), meropenem (10 µg), and imipenem (10 µg). Escherichia coli ATCC 25922 was selected as the quality control strain in this test. The results were interpreted according to the CLSI guidelines. Double-disc Synergy Test (DDST) was performed to confirm ESBL production phenotypically. In this experiment, the ceftazidime and cefotaxime discs were placed at a distance of 15 mm from the amoxicillin/clavulanic acid disc.
3.4. Evaluation of ESBL and Carbapenemase-Encoding Genes
The studied beta-lactamase genes were identified by the PCR method. The presence of ESBL-encoding (blaCTX-M1, blaSHV, and blaTEM) and carbapenemase-encoding (blaKPC, blaOXA-48, blaNDM, blaVIM, and blaIMP) genes was investigated. These genes were detected by specific primers (17-24). DNA extraction was performed by the boiling method.
3.5. Statistical Analysis
Statistical analysis was performed using SPSS version 23 software. The chi-square test assessed the relationship between ESBL carriage (dependent variable) and risk factors, including sex, age, occupation, education, diabetes history, gastrointestinal status, antibiotic consumption, and the number of dialysis sessions (independent variables). Univariate and multivariate analyses were performed using logistic regression. In multivariate analysis, ESBL carriage was the dependent variable, and related factors were the independent variables. In this analysis, factors with a P-value of < 0.05 were considered statistically significant.
4. Results
4.1. Study Population
This study was performed on 150 hemodialysis patients, including 52 women and 98 men. The distribution of patients based on the number of dialysis years was as follows: 0 - 2 years, 2 - 4 years, 4 - 7 years, and more than seven years. Also, they were divided into four age groups: 18 - 42, 43 - 54, 55 - 67, and over 67 years. Furthermore, 53 out of 150 patients had diabetes, while 25 were carriers of ESBL-producing bacteria.
4.2. Identification of Isolates and Antibiotic Susceptibility Testing
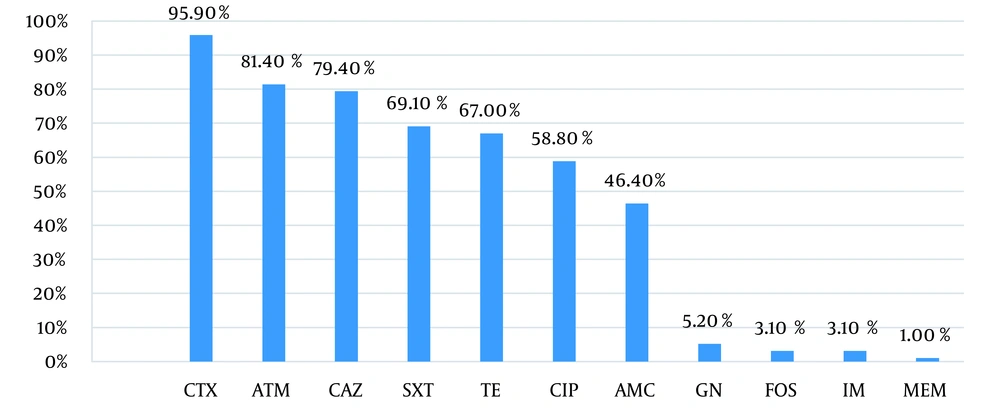
In this study, 106 bacterial isolates were collected from 73 out of 150 patients studied. The biochemical tests identified the bacteria as follows: E. coli (90.6%, n = 97), K. pneumoniae (3.73%, n = 4), Enterobacter cloacae (0.93%, n = 1), K. oxytoca (0.93%, n = 1), and Morganella morganii (2.83%, n = 3). Infection to more than one type of bacteria was found in five out of 150 patients (Table 1). The rates of resistance to cefotaxime (CTX 30 µg), ceftazidime (CAZ 30 µg), aztreonam (ATM 30 µg), trimethoprim-sulfamethoxazole (SXT 1.25 - 23.75 µg), and tetracycline (TE 30 µg) were more than 70%. Also, the highest susceptibility to gentamicin, imipenem, fosfomycin, and meropenem was observed in 100, 101, 101, and 106 isolates, respectively (Figure 1, Table 2). In this study, multidrug-resistant (MDR) was the antimicrobial resistance of a microorganism to at least one antimicrobial drug in three or more antimicrobial categories, except for aztreonam. The prevalence of MDR isolates was 73.8%.
| Type of Bacteria | Number of Patients |
|---|---|
| Escherichia coli + Klebsiella pneumoniae | 2 |
| E. coli + K. oxytoca | 1 |
| E. coli + Enterobacter cloacae | 1 |
| E. coli + Morganella morganii | 1 |
| Antimicrobial Agent | Escherichia coli (N = 97) | Klebsiella pneumoniae (N = 4) | K. oxytoca (N = 1) | Enterobacter cloacae (N = 1) | Morganella morganii (N = 3) |
|---|---|---|---|---|---|
| Amoxicillin/clavulanic acid | 46.4 | 50 | 0 | 25 | 25 |
| Tetracycline | 67 | 25 | 0 | 0 | 75 |
| Gentamicin | 5.2 | 25 | 0 | 0 | 25 |
| Aztreonam | 81.4 | 50 | 0 | 0 | 0 |
| Cefotaxime | 95.9 | 75 | 0 | 25 | 75 |
| Ceftazidime | 79.4 | 50 | 0 | 25 | 75 |
| Ciprofloxacin | 58.8 | 25 | 0 | 0 | 50 |
| Fosfomycin | 3.09 | 25 | 0 | 0 | 0 |
| Trimethoprim/sulfamethoxazole | 69.1 | 0 | 25 | 0 | 0 |
| Imipenem | 3.1 | 0 | 25 | 0 | 0 |
| Meropenem | 1.0 | 0 | 0 | 0 | 0 |
4.3. Phenotypic and Genotypic Detection of ESBLs
The DDST was performed on 106 resistant isolates to phenotypically confirm the production of ESBL, which resulted in the identification of 70 (67.3%) out of 106 isolates as phenotypically approved ESBL producers. In two DDST-positive isolates, no ESBL genes were detected by the PCR method, while 28 out of 36 unapproved isolates in the DDST method carried the ESBL genes studied. Also, only three isolates showing resistance to carbapenems in the DDST were confirmed directly by the PCR method.
4.4. Genotypic Characteristics of ESBL and Carbapenemase
The CTX gene was detected in 82 isolates as the dominant ESBL gene. The TEM and SHV genes were detected in 26.4% and 24.5% of the isolates, respectively. Also, 17 E. coli isolates carried a combination of the CTX and SHV genes. In addition, 17 E. coli and one K. pneumoniae isolates carried a combination of the CTX and TEM genes. Co-harboring of SHV and TEM genes was found in one E. coli isolate (Table 3). The OXA-48 gene was found in 4.6% of the isolates, including two E. coli and two M. morganii isolates. Unexpectedly, the KPC gene was only detected in 12 E. coli isolates (Table 4). None of the VIM, IMP, and NDM genes were detected in CPE isolates. Furthermore, the co-harboring of carbapenemase genes was not found in any of the isolates. Instead, 14.06% (n = 15) of the isolates carried a combination of both ESBL and carbapenemase genes.
| Species | No. of Isolates (%) | SHV | TEM | CTX | CTX -SHV | CTX- TEM | SHV-TEM |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 97 (91.5) | 24 | 26 | 79 | 17 | 17 | 1 |
| Klebsiella pneumoniae | 4 (3.7) | 2 | 1 | 1 | - | 1 | - |
| K. oxytoka | 1 (0.9) | - | - | - | - | - | - |
| Enterobacter cloacae | 1 (0.9) | - | - | - | - | - | - |
| Morganella morganii | 3 (2.8) | - | - | - | - | 1 | - |
| Species | No. of Isolates (%) | OXA-48 | KPC | VIM | IMP | NDM |
|---|---|---|---|---|---|---|
| Escherichia coli | 97 (91.5) | 2 | 12 | - | - | - |
| Klebsiella pneumoniae | 4 (3.7) | - | - | - | - | - |
| K. oxytoka | 1 (0.9) | - | - | - | - | - |
| Enterobacter cloacae | 1 (0.9) | - | - | - | - | - |
| Morganella morganii | 3 (2.8) | 2 | - | - | - | - |
4.5. Risk Factors and Prevalence of Rectal Carriage of ESBL in Enterobacteriaceae
The effect of risk factors on the intestinal carriage of ESBL was investigated by univariate and multivariate statistical analyses (Table 5). The patients were divided into four age groups to evaluate the effect of age on the fecal carriage of ESBL. According to multivariate analysis, there was a significant association between the age group of 18 - 42 years and ESBL carriage (P = 0.04; OR = 0.32; 95%CI: 0.10 - 0.99). Moreover, patients were divided into four groups to investigate the effect of dialysis sessions. There was no significant relationship between the number of dialysis sessions and ESBL carriage in either analysis. Diabetes was surveyed as an important possible risk factor in carriers. This factor had no significant relationship with ESBL carriage (univariate: P = 0.71, OR = 0.88, 95%CI: 0.45 - 1.72) (multivariate: P = 0.66, OR = 0.84, 95%CI: 0.39 - 1.80). Occupation and education were the other risk factors examined in this survey. Neither of these risk factors was significantly associated with ESBL carriage. The status of the gastrointestinal tract, which is affected by diet, was examined as another possible risk factor in these patients. Accordingly, patients were divided into three groups: Constipation, normal, and diarrhea. There was no significant relationship between any of the groups and ESBL carriage. The use of antibiotics was examined as an important risk factor considered in various studies worldwide. No link was found between this factor and ESBL carriage.
| Variables | Total No. | No. (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| P-Value | OR | P-Value | OR | |||
| Sex | ||||||
| Male | 98 | 53 (54.1) | 0.07 | 1.88 (0.94 - 3.74) | 0.23 | 1.63 (0.73 - 3.62) |
| Female | 52 | 20 (38.5) | 1 | 1 | ||
| Age | ||||||
| 18 - 42 | 37 | 16 (43.2) | 0.064 | 0.41 (0.16 - 1.05) | 0.03 | 0.28 (0.09 - 0.88) |
| 43 - 54 | 39 | 17 (43.6) | 0.065 | 0.41 (0.16 - 1.05) | 0.08 | 0.40 (0.14 - 1.11) |
| 55 - 67 | 37 | 16 (43.2) | 0.064 | 0.41 (0.16 - 1.05) | 0.05 | 0.37 (0.13 - 1.03) |
| > 67 | 37 | 24 (64.9) | 1 | 1 | ||
| Number of dialysis years | ||||||
| 0 - 2 | 36 | 13 (36.1) | 0.20 | 0.53 (0.19 - 1.41) | 0.73 | 0.82 (0.27 - 2.52) |
| 2 - 4 | 37 | 18 (48.6) | 0.80 | 0.88 (0.34 - 2.30) | 0.71 | 1.22 (0.41 - 3.57) |
| 4 - 7 | 46 | 26 (56.5) | 0.67 | 1.21 (0.48 - 3.04) | 0.27 | 1.79 (0.63 - 5.09) |
| > 7 | 31 | 16 (51.6) | 1 | 1 | ||
| Occupation | ||||||
| Self-employed | 16 | 11 (68.8) | 0.09 | 2.55 (0.84 - 7.75) | 0.11 | 2.79 (0.76 - 10.13) |
| Salaried | - | - | - | - | ||
| Unemployed | 134 | 62 (46.3) | 1 | 1 | ||
| Education | ||||||
| High school | 88 | 41 (46.6) | 0.79 | 0.87 (0.31 - 2.04) | 0.41 | 0.62 (0.19 - 1.94) |
| Diploma | 44 | 23 (52.3) | 0.87 | 1.09 (0.36 - 3.28) | 0.83 | 1.13 (0.32 - 3.97) |
| Above diploma | 18 | 9 (50.0) | 1 | 1 | ||
| Antibiotic consumption | ||||||
| No consumption | 138 | 69 (50) | 0.12 | 3.50 (0.70 - 17.4) | 0.12 | 4.03 (0.68 - 23.7) |
| Previous consumption | 3 | 2 (66.7) | 0.18 | 7.00 (0.39 - 123.3) | 0.17 | 8.24 (0.38 - 178.6) |
| Consumption at the time of sampling | 9 | 2 (22.2) | 1 | 1 | ||
| Digestive status | ||||||
| Constipation | 35 | 18 (51.4) | 0.80 | 1.09 (0.51 - 2.35) | 0.79 | 1.14 (0.45 - 2.87) |
| Diarrhea | 7 | 2 (28.6) | 0.30 | 0.41 (0.07 - 2.23) | 0.43 | 0.50 (0.87 - 2.86) |
| Normal | 108 | 53 (49.1) | 1 | 1 | ||
| Diabetes mellitus | ||||||
| Diabetic | 53 | 25 (47.2) | 0.78 | 0.91 (0.46 - 1.78) | 0.74 | 0.88 (0.41 - 1.87) |
| Non-diabetic | 97 | 48 (49.5) | 1 | 1 | ||
5. Discussion
Evaluation of intestinal colonization with ESBL-E and CPE is of great importance because of the increased risk of bacterial infections. In Iran, a few studies have been performed on the fecal carriage of CPE and ESBL-PE. In a study conducted by Aghamohammad et al. in Iran, the prevalence rate of fecal carriage of ESBL-PE was 18.3% among intensive care unit patients and outpatients (25). In another study by Bazargani and Hashemzade in Iran, the prevalence rate of ESBL carriage among hospitalized patients with diarrhea was 25.9% (26). Fecal carriage of ESBL among patients with urinary tract infection was reported to be 35% in another study from Kerman (27). In this study, for the first time, the prevalence of intestinal carriage of ESBL-PE and CPE and its related risk factors were investigated among hemodialysis patients. The incidence of the fecal carriage was estimated to be high among these patients due to frequent hospital visits, frequent contact with staff and medical devices, hospital food intake, and the use of antibiotics. Recent studies have reported different prevalence rates for ESBL carriage, including 1.9%, 30%, and 52.1% (28-30).
As expected, in the present study, a high prevalence rate was obtained for ESBL carriage among hemodialysis patients (75 of 150 patients). However, only 2% of the study population were identified as CPE carriers. More than 50% of ESBL-producing isolates were resistant to ciprofloxacin, tetracycline, trimethoprim/sulfamethoxazole, ceftazidime, aztreonam, and cefotaxime. Simultaneous harboring of genes encoding resistance to different antibiotics on the same mobile genetic element is one of the main reasons for the emergence of MDR isolates. Although these genes were not the main subject of the study, the PCR method showed that blaCTX-M and blakpc genes were the predominant genes encoding ESBL and carbapenemase, respectively. Moreover, five participants carried two different bacterial isolates. These findings are probably due to the inter-species transfer of varying resistance genes by mobile genetic elements between different bacterial strains, which could also spread across the intestinal ecosystem (31-33).
Various studies have been conducted worldwide to examine the prevalence of ESBL-E and CPE and their related risk factors (34, 35). Identifying the risk factors associated with intestinal colonization is a way to control and prevent the spread of antibiotic resistance. In some studies, intestinal flora has been introduced as a risk factor for resistance (28, 36, 37). The prevalence of fecal carriage of ESBL has been reported to vary across different age groups (38-41). However, the impact of age on fecal carriage varies with the age range of the study populations. Tellevik et al. reported age less than 12 months as a risk factor for ESBL carriage (42). In another study, Cakir Erdogan et al. demonstrated age greater than 65 years as a related factor for intestinal carriage of ESBL (28). However, in this study, patients aged 18 - 42 years were more likely to be colonized by ESBL-producing Enterobacteriaceae than those in the other age groups. There were different results about the association between sex and ESBL carriage. A study by Pitout et al. showed that the rate of rectal carriage of ESBL bacteria was higher among females (37). In contrast, Ben-Ami proved that males were more prone to be an ESBL-producing bacteria carrier (36). However, sex was not recognized as a risk factor in the present study.
The role of diabetes mellitus as a risk factor for fecal carriage of ESBL has been proven in many studies (28, 30, 43). Diabetic people are more prone to bacterial infections due to their weakened immune systems. Schoevaerdts et al. showed that the bacteria isolated from the urethra of diabetic patients with urinary tract infections were the same as the bacteria colonizing the intestines of these patients (44). This finding is inconsistent with the present study, in which no significant association was found between diabetes and fecal carriage. Unemployment can also be associated with an increased carriage rate. Poor hygiene and non-compliance with hygienic principles due to poor economic conditions can increase ESBL colonization (45). However, occupation was not recognized as a risk factor affecting ESBL carriage in this study. In a study from India, highly educated people were more prone to intestinal colonization with resistant bacteria. It was hypothesized that this group might also be socio-economically better off, resulting in more likely seeking health care and higher exposure to antibiotics (46). While the carriage rate was lower among educated individuals in Turkey (28), no association was found between education level and resistant intestinal flora in the present study.
Furthermore, it was hypothesized that patients with a longer history of dialysis were more likely to develop resistant gut flora due to more hospital visits. Although the number of carriers with a history of four to seven years of dialysis was higher than the number of carriers in other groups, no significant relationship was found between the number of dialysis sessions and fecal colonization. The commensal flora of patients in the hospital is replaced by pathogenic bacteria under selective pressure due to factors such as the overuse of antibiotics. Antibiotic use in intensive care unit patients was a risk factor in the studies by Solgi et al. in Iran and Day et al. in India (32, 47). The rate of colonization was also high among antibiotic users admitted to a hospital in Pakistan (47). In the present study, although antibiotic users were carriers, statistical analysis showed no significant relationship between antibiotic use and resistant bacteria carriage.
5.1. Conclusions
This study highlights the high fecal carriage of ESBL-producing Enterobacteriaceae among hemodialysis patients. This indicates that these are expanding in our hospital setting. The high rate of resistance to many classes of antibiotics has caused concern due to therapeutic problems and the risk of transmitting resistance genes to other patients. Understanding the factors associated with intestinal colonization will help prevent infection. Carriage is a circulating pool of resistance genes leading to disease. Therefore, the link between carriage and infection needs further elucidation.