Dear Editor,
The COVID-19 pandemic is not over. Since 2019, persistently augmenting SARS-CoV-2 strains are causing millions of deaths worldwide (1). Although multiple COVID-19 vaccines are commercially available, the rate of mass vaccination is very low due to the lack of mega-scale production capability and limited supply. Also, among poor countries, an acute shortage of SARS-CoV-2 vaccines has become a significant public health issue, causing a rise in ongoing viral pandemics and the rise of multiple variants (2-5). Most commercially available vaccines have been strategized to induce neutralizing antibodies against the spike region of SARS-CoV-2 to block early-stage viral infection. However, these strategies need to be revised and upgraded due to rapidly evolving strains of the virus (6, 7).
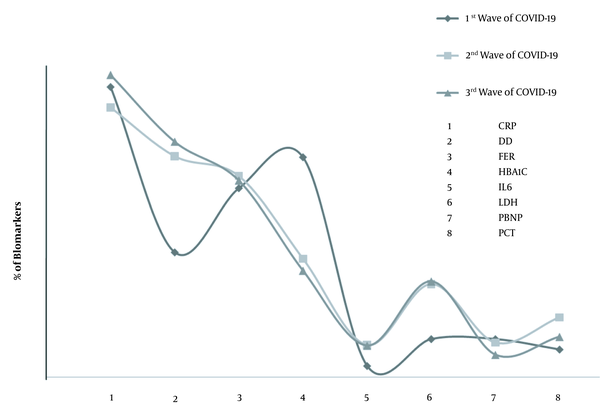
In Pakistan, 1.285 million people were infected with SARS-CoV-2, among whom 28,745 individuals died of COVID-19. As of second December 2021, 124,054,300 COVID-19 vaccine doses were administered to the general public aged 12 to 60 years and above (8-12). Herein, we examined the clinical association of biomarkers among SARS-CoV-2-infected people. The abnormal biomarkers linked with SARS-CoV-2 during the first three waves of COVID-19 in Pakistan were investigated during the study. The study was conducted at Islamabad Diagnostic Center (IDC) to analyze COVID-19 infections from first April 2020 to 31 July 2020 (encompassing the first wave of COVID-19), first August 2020 to 31 January 2021 (encompassing the second wave of COVID-19), and first February 2021 to 15 July 2021 (encompassing the third wave of COVID-19). The real-time PCR-confirmed samples were analyzed using the standardized protocols and test kits, as described previously (2), to determine C-reactive protein (CRP), D-dimer (DD), ferritin (FER), hemoglobin A1c (HBA1c), interleukin 6 (IL6), lactate dehydrogenase (LDH), NT-pro-B-type natriuretic peptide (PBNP), and procalcitonin (PCT). This study was approved by the Institutional Review Board of IDC, Pakistan. During the first three waves of COVID-19, 789,998 suspected cases of SARS-CoV-2 were selected.
Among the included subjects, 11.2% were from the first wave of COVID-19 (April - July 2020), 55.4% were from the second wave of COVID-19 (August 2020 - January 2021), and 33.3% were from the third wave of COVID-19 (February - July 2021). Among 516,178 suspected subjects, the overall prevalence rate of SARS-CoV-2 was 12% during 2019-2021 in Pakistan. Among real-time PCR-positive cases, 21.35, 32.5, and 46.15% belonged to the first, second, and third waves of SARS-CoV-2 in Pakistan. During the first wave of COVID-19, among SARS-CoV-2-positive cases, 28.1% of CRP, 12.1% of DD, 18.3% of FER, 21.3% of HBA1c, 1.1% of IL6, 11.6% of LDH, 3.7% of PBNP, and 3.69% of PCT biomarkers were evaluated. Interestingly, based on radiological findings, 2.83% of the subjects were positive for high-resolution computed tomography (HRCT) and the aforementioned biomarkers.
During the second wave, among SARS-CoV-2-infected subjects, 26.14% of CRP, 21.42% of DD, 19.5% of FER, 11.53% of HBA1c, 3.14% of IL6, 9.04% of LDH, 3.37% of PBNP, and 5.82% of PCT biomarkers were evaluated. The results showed that 6.03% of the subjects infected in the second wave of COVID-19 were positive for HRCT and the aforementioned biomarkers. Among SARS-CoV-2-infected subjects in the third wave, 29.3% of CRP, 22.82% of DD, 19.06% of FER, 10.33% of HBA1c, 3.06% of IL6, 9.29% of LDH, 2.171% of PBNP, and 3.92% of PCT biomarkers were analyzed. Of note, 12.65% of SARS-CoV-2-positive cases in the third wave of COVID-19 were positive for HRCT and the aforementioned biomarkers. The analysis revealed that 30.67% of SARS-CoV-2 PCR-positive patients depicted abnormal HBA1c, 51.5% had abnormal DD, and 25.04% had abnormal CRP biomarkers.
Compared to the first wave of COVID-19, during the second and third waves, the tendency to DD tests was enhanced by 1.7 and 1.88 folds, respectively. Similarly, in contrast to the first wave, IL6 enhanced by 2.85 and 2.78 folds during the second and third waves, respectively. Also, the levels of LDH were enhanced by 2.44 folds during the second wave and 2.5 folds during the third wave of COVID-19, as shown in Figure 1. Of note, during the third wave of COVID-19, the CO-RADS score analysis among patients with abnormal CRP, DD, FER, HBA1C, IL6, LDH, PBNP, and PCT biomarkers revealed that 25.082% of the patients had mild HRCT, 47.8% had moderate HRCT, and 27.07% had severe HRCT status.
The current study is critical not only for physicians across the world but also for health reforming strategic organizations at the international level. The current study revealed that during SARS-CoV-2 infection, CRP, DD, FER, HBA1C, IL6, LDH, PBNP, and PCT biomarkers showed a positive association with mild-moderate and severe HRCT status depending on the SARS-CoV-2 waves during 2019-2021. It also urges the need for further in-depth analysis of biomarkers associated with SARS-CoV-2. Due to limitations, we could not further investigate the effects of these biomarkers on viral replication and their role in intracellular signaling. Furthermore, it would be interesting to determine the impact of these biomarkers in both pre- and post-SARS-CoV-2 vaccinated people. Also, this study would further open doors of investigation among persons with repeated histories of COVID-19 infections.