1. Background
About 70% of individuals worldwide suffer from herpes simplex virus 1 (HSV-1). It can lead to a variety of clinical problems affecting the genital tract, eyes, skin, and central nervous system (1). A crucial structural protein in the HSV-1 virion tegument is unique long 47 (UL47) (or VP13/VP14). It is a ribonucleic acid (RNA)-binding protein that moves back and forth between the cytoplasm and nucleus. According to studies showing that recombinant UL47 mutant viruses exhibit decreased growth in cell cultures and less pathogenicity in a mouse model, it has been proposed that UL47 might be a beneficial regulator of viral replication and pathogenicity (2). The UL47 promotes the replication of HSV-1 through interaction with some viral proteins (e.g., infected cell culture polypeptide 27 (ICP27) and unique long 17 (UL17)) and regulation of their subcellular localization. Additionally, it participates in the initial virions’ envelope formation in the perinuclear region, most likely through interactions with the nuclear egress proteins UL31 and UL34 (3).
The HSV-1 is also the most common cause of sporadic life-threatening encephalitis in developed countries. The HSV-1-related lesions are treated using commercially available antivirals, such as acyclovir, valacyclovir, and penciclovir. However, immunosuppressed patients are more likely than immunocompetent individuals to develop drug resistance after receiving prolonged treatment. Resistance to acyclovir is associated with mutations in viral enzymes, namely thymidine kinase and deoxyribonucleic acid (DNA) polymerase (4). Nephrotoxicity is a side effect of several second-line alternatives, such as cidofovir and foscarnet (5). Probiotics are live microorganisms that support the health of the host. Common probiotic species include Bacillus, Saccharomyces, Bifidobacterium, Streptococcus, and Lactobacillus (6).
Numerous antibiotic metabolites produced by the Bacillus genus, including bacteriocins, lipopeptides, glycopeptides, and cyclic peptides, can inhibit some infections. The antiviral activity of several bacilli has been demonstrated against human viruses, including Bacillus subtilis against the influenza virus and herpes simplex virus 2 (HSV-2) (7), B. horneckiae against HSV-1, and Bifidobacterium licheniformis against HSV-2 (7). In addition, B. pumilus ribonuclease (binase) has been introduced as an inhibitor of influenza A and rhinovirus serotype 1A in cell culture (8). Although B. clausii is known as a probiotic, its antiviral activity is unclear (9). A study has reported that B. clausii has antiviral activity against rotavirus (7).
Infections with viruses, such as HSV-1, influenza, vesicular stomatitis, Epstein-Barr virus, and human immunodeficiency virus (HIV), frequently result in the production of reactive oxygen species (ROS) (10). For numerous biological processes, ROS can be both helpful and harmful, and an excess of ROS causes oxidative stress. In many cases, ROS can increase viral replication. Selenium (Se) has an important role in antioxidant defense. An essential antioxidant enzyme called glutathione peroxidase requires selenium as a cofactor. Glutathione peroxidase’s activity is reduced by a selenium deficit. According to several studies, a selenium deficit encourages viral mutations, replication, and the generation of viruses with higher dangerous potential (11, 12). Researchers suggest that selenium can play a role in cell signaling and attachment of the herpes virus; therefore, various herpes viruses are responsive to selenium therapy. In a study conducted on bovine herpes virus type 1 (BHV-1), selenium inhibited viral replication and reduced its cytopathic effect (CPE) (13).
2. Objectives
It is unknown whether B. clausii metabolites have any effect on human viruses, and further research is necessary. Additionally, it is unknown how selenium affects HSV-1 replication in vitro. The current study aimed to assess the impact of selenium, B. clausii supernatant, and their combination on HSV-1.
3. Methods
3.1. Cell Line and Virus
The human herpes virus 1 and HeLa cells were provided by the Pasteur Institute of Iran. HeLa cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma, USA) containing 10% fetal calf serum (Sigma, USA) and 1% Penicillin-Streptomycin (10,000 U/mL, Gibco, Germany). The HSV-1 was cultured in HeLa cells. Four days after the infection of the cells, the virus was released from the cells following three freeze-thaw cycles.
3.2. Bacillus clausii Supernatant
The Iranian Biological Resource Center in Tehran, Iran, provided the B. clausii strain in lyophilized form (ATCC 700160). Bacillus clausii supernatant was prepared by growing 106 CFU/mL of bacteria in 40 mL of tryptic soy broth medium and incubation at 37°C for 24 hours. After centrifuging the microbial cultures at 3000 rpm for 20 minutes, the supernatants were filtered (0.22 μM syringe filter) and collected. The supernatant was neutralized and adjusted (pH 7.2) due to its acidic nature before usage.
3.3. Cytotoxicity Assay of the Bacterial Supernatant and Selenium
For the assessment of the cytotoxicity of B. clausii supernatant and selenium, HeLa cells at a density of 2 × 104 cells/well in triplicate were seeded into a 96-well plate. Cell viability was assessed using the MTT (dimethyl thiazolyl-diphenyl tetrazolium bromide) test. One hundred μL of each serial dilution of bacterial supernatant (up to 1:128) or selenium (from 0.5 μM to 8 μM) was added to cell monolayers and incubated for 24 hours at 37°C with 5% CO2. The cells with DMEM (without bacterial supernatant) were used as a negative control. After three phosphate-buffered saline washes, 90 μL of fresh DMEM and 10 μL of MTT (BIO-IDEA, Iran) were added to each well. The microplates were incubated for 4 hours at 37°C. Dimethyl sulfoxide was added to the cells, and an ELISA reader was used to measure the absorbances at 570 nm. The following formula was used to calculate the percentage of viable cells:
(A treatment/A control) × 100%
GraphPad Prism software (version 9.0; San Diego, USA) was used to calculate the 50% cytotoxic concentration.
3.4. Titration of HSV-1
The HSV-1 titer was measured using the 50% tissue culture infectious dose (TCID50) technique, as previously published (14). A seeding density of 104 cells per well was used to cultivate HeLa cells in 96-well plates for 24 hours until 80% confluency was obtained. The virus stock was serially diluted 10 times (10-1 - 10-9) in DMEM, and the 96-well plate’s wells received 100 ul of each dilution. For each dilution, four duplicates were carried out. Furthermore, eight wells of each microplate were used as cell controls with no virus dilutions. The microplates were incubated at 37°C with 5% CO2 for 10 days, and the CPE of HSV-1 was measured daily with an inverted microscope. The number of wells in which the CPE from each dilution was observed was counted. The Karber formula was used to calculate virus titers by measuring the TCID50.
3.5. Antiviral Activity Assay in Various Conditions
3.5.1. Pre-infection Treatment Assay
In 6-well plates, HeLa cell monolayer cultures were grown to confluence. The cells were treated with selenium, B. clausii supernatant, and a combination of them and then infected with HSV-1 after 24 hours of incubation at 37°C. The titer of viruses and UL47 transcript levels were examined after 78 hours of virus inoculation.
3.5.2. Post-infection Treatment Assay
The HeLa cell monolayer cultures were developed to confluence in 6-well plates. The cells were infected by HSV-1. After 24 hours of incubation at 37°C, the cells were treated with selenium, B. clausii supernatant, or a combination of them. The titer of viruses and UL47 transcript levels were examined after 48 hours of virus inoculation. After the determination of the titer of the virus in the presence of different dilutions of selenium, B. clausii supernatant, or a combination of them, GraphPad Prism software (version 9.0) was used to determine the median effective concentration (EC50) (15).
3.6. Relative Quantification of HSV-1 UL47 by Real-Time Polymerase Chain Reaction
The TCID50 results were approved by measuring HSV-1 UL47 levels in different experimental assays. Total RNA was extracted from cultured cells using the RNX plus (SinaClon, Iran) by the manufacturer’s instructions. Using the AddScript cDNA Synthesis Kit (AddBio, South Korea), reverse transcription was performed on 1 μg of total RNA. ABI StepOnePlus™ instrument (ABI, USA) was used to perform a real-time polymerase chain reaction (PCR) assay using a RealQ Plus 2x Master Mix Green (Ampliqon, Denmark) and specific primers (UL47-forward: 5’-GTTACCGGATTACGGGGACT-3’, UL47- reverse: 5’-GACGTACGCGATGAGATCAA-3’, β-globin-forward: GGCGGCACCACCATGTACCCT, and β-globin-reverse: AGGGGCCGGACTCGTCATACT). Each PCR mixture comprised 12.5 μL of master mix (Ampliqon, Denmark), 2 μL of cDNA, 0.5 μL (10 μmol) of primer, and 9.5 μL of distilled water. The cycling profile, including initial denaturation for 10 minutes at 94°C, followed by 40 cycles of denaturation for 15 seconds at 95°C, annealing for 30 seconds at 60°C, and elongation for 30 seconds at 72°C, was used for amplification. After normalizing the data against the expression of human β-globin, the relative expression levels of UL47 were calculated using the 2−ΔΔCT method.
3.7. Statistical Analysis
Real-time PCR and TCID50 technique results were compared between the two groups, and significant differences were determined using GraphPad Prism software (version 8.0). Statistical significance was defined as a p-value of 0.05 or less. Each experiment was carried out three times, and the results were reported as mean and standard deviation.
4. Results
4.1. Cytotoxicity Assay of the Bacterial Supernatant and Selenium
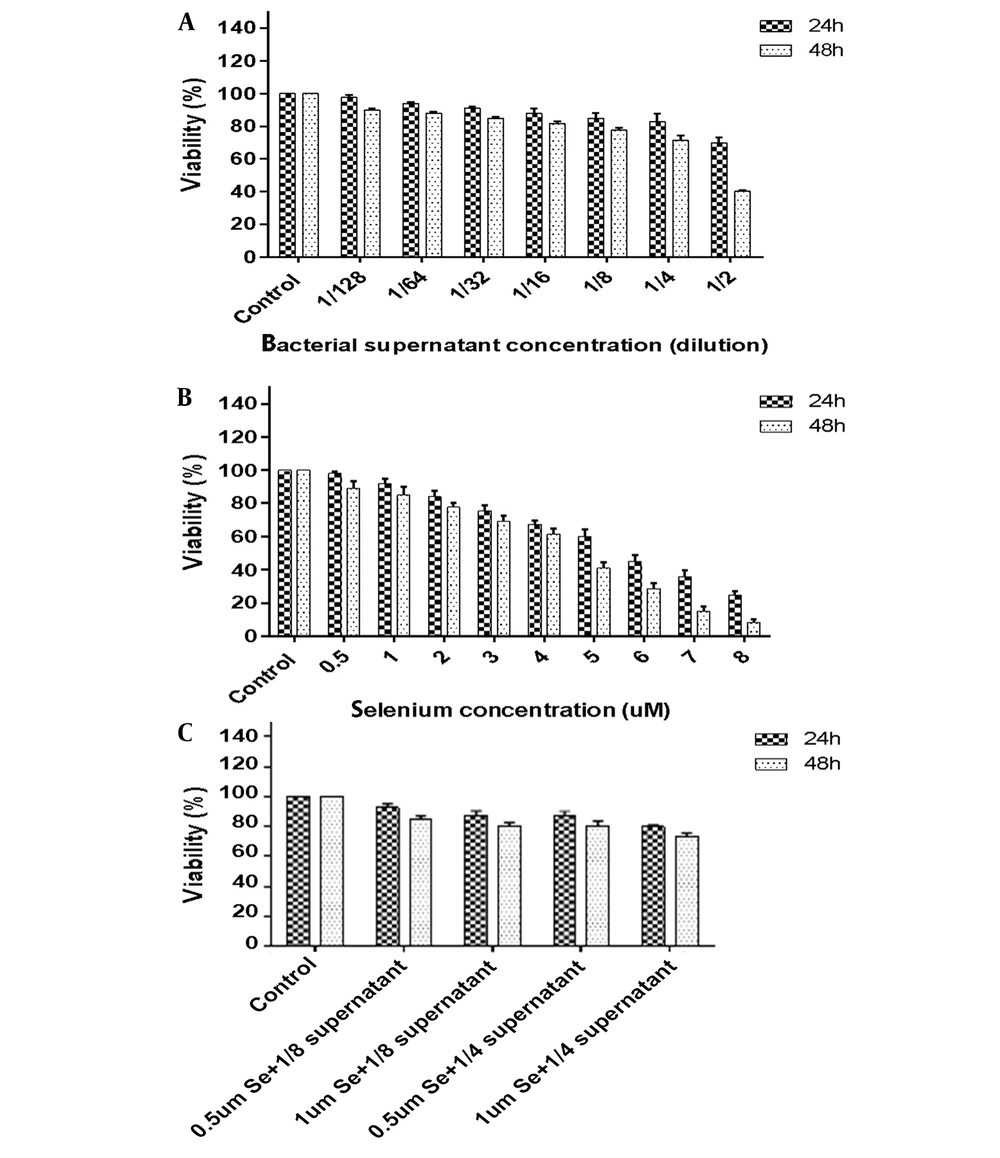
Figure 1 depicts the impacts of B. clausii supernatant, selenium, and a combination of them on HeLa cell viability. The results of the MTT assay showed that the treatment of HeLa cells with the bacterial supernatant at a dilution of 1/2 during 24 and 48 hours decreased cell viability to 65% and 40%, respectively. Moreover, when HeLa cells were exposed to the bacterial supernatant at dilutions lower than 1/4 for 24 hours and lower than 1/8 for 48 hours, 80% of the cells were viable. Selenium with concentrations of 0.5 to 4 μM had cytotoxicity lower than 50% during 24 and 48 hours of incubation. Figure 1 illustrates the synergistic effect of selenium and bacterial supernatant on cell viability. A cytotoxicity level of lower than 60% was observed when 0.5 or 1 μM selenium was combined with 1/4 and 1/8 dilutions of bacterial supernatant.
4.2. Evaluation of HSV-1 Titer Reduction by TCID50 in Various Conditions of Treatment
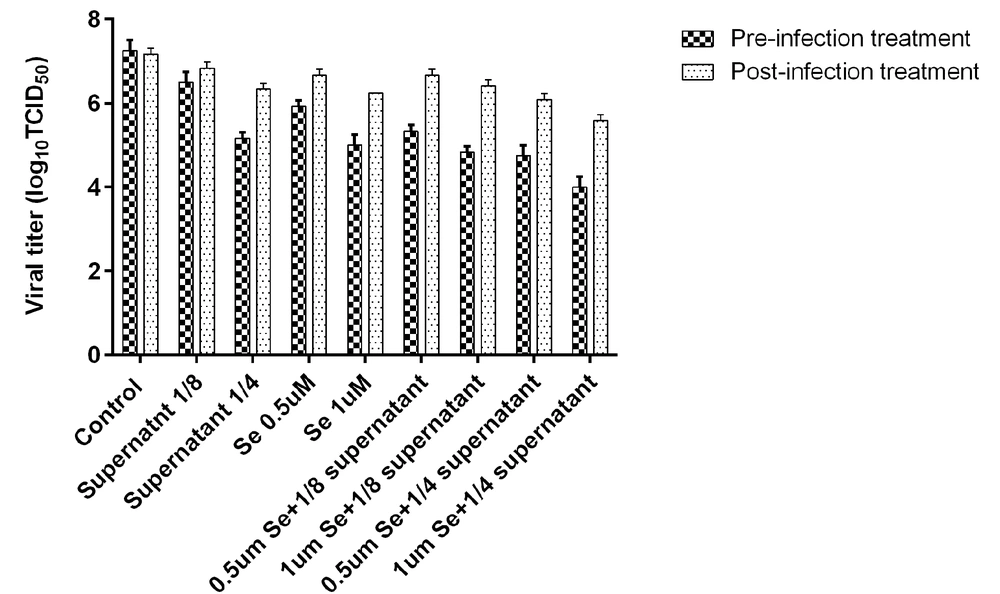
After the incubation of bacterial supernatant (dilution 1/4 and 1/8) and Se (0.5 and 1 μM) before virus inoculation (pre-infection) or after virus inoculation (post-infection), the titer of HSV-1 was estimated (Table 1, Figure 2). The HSV-1 titer in all experimental assays was significantly lower than the virus titer in the control group. In general, the reduction of the virus titer in the post-infection assay was lower than in the pre-infection assay. The HSV-1 titers in pre-infection and post-infection assays with a dilution of 1:4 supernatant decreased by about 2.16 and 1 log10 TCID50/mL in comparison to the control, respectively. Se in a concentration of 1 μM could reduce the virus titer to about 2.33 log10 TCID50/mL.
The virus titer showed a greater decrease when selenium and bacterial supernatant were combined than when only one of the two was used. The combined use of 1 μM Se and 1/4 dilution of B. clausii supernatant caused the greatest drop in virus titer (3.3 log10 TCID50/mL) in comparison to other treatment conditions (Table 1, Figure 3). Table 2 shows a summary of 50% cytotoxicity concentration, EC50, and selectivity index (SI) of selenium and B. clausii supernatant. In general, the SI was higher in pre-infection assays than in post-infection assays. In post-infection assays, selenium and bacterial supernatant had an SI lower than 10. The highest SI was obtained when selenium and bacterial supernatant were combined. The mixture of bacterial supernatant and 0.5 μM selenium had an SI value of 13.6. The combination of bacterial supernatant with 1 μM selenium had an SI value of 29.2 (Table 2).
| Treatment | Pre-infection | Post-infection | ||
|---|---|---|---|---|
| Mean of Log10 TCID50/mL ± SD | ||||
| Virus Titer | Decreased Titer | Virus Titer | Decreased Titer | |
| Control | 7.33 ± 0.14 | - | 7.17 ± 0.14 | - |
| Supernatant 1:8 | 6.50 ± 0.25 | -0.83 | 6.83 ± 0.14 | -0.5 |
| Supernatant 1:4 | 5.17 ± 0.14 | -2.16 | 6.33 ± 0.14 | -1 |
| Se 0.5 μM | 5.92 ± 0.14 | -1.41 | 6.67 ± 0.14 | -0.66 |
| Se 1 μM | 5.00 ± 0.25 | -2.33 | 6.25 | -1.08 |
| 0.5 μM Se + 1:8 supernatant | 5.33 ± 0.14 | -2 | 6.67 ± 0.14 | -0.66 |
| 1 μM Se + 1:8 supernatant | 4.83 ± 0.14 | -2.5 | 6.42 ± 0.14 | -0.91 |
| 0.5 μM Se + 1:4 supernatant | 4.75 ± 0.25 | -2.58 | 6.08 ± 0.14 | -1.25 |
| 1 μM Se + 1:4 supernatant | 4.00 ± 0.25 | -3.33 | 5.58 ± 0.14 | -1.75 |
Abbreviations: TCID50, 50% tissue culture infectious dose; SD, standard deviation.
| Compound | Pre-infection Assay | Post-infection Assay | ||||
|---|---|---|---|---|---|---|
| CC50 | EC50 | SI (CC50/EC50) | CC50 | EC50 | SI (CC50 /EC50) | |
| Selenium (μM /mL) | 5.4 | 0.8 | 6.75 | 5.4 | 2.4 | 2.25 |
| Supernatant (dilution) | 0.45 | 0.12 | 3.75 | 0.45 | 0.27 | 1.66 |
| Supernatant + 0.5 μM selenium | 0.41 | 0.03 | 13.66 | 0.41 | 0.21 | 1.95 |
| Supernatant + 1 μM selenium | 0.35 | 0.012 | 29.2 | 0.35 | 0.11 | 3.2 |
Abbreviations: CC50, 50% cytotoxicity concentration; EC50, median effective concentration; SI, selectivity index.
4.3. Results of Relative Quantification of UL47 Gene in Various Conditions of Treatment
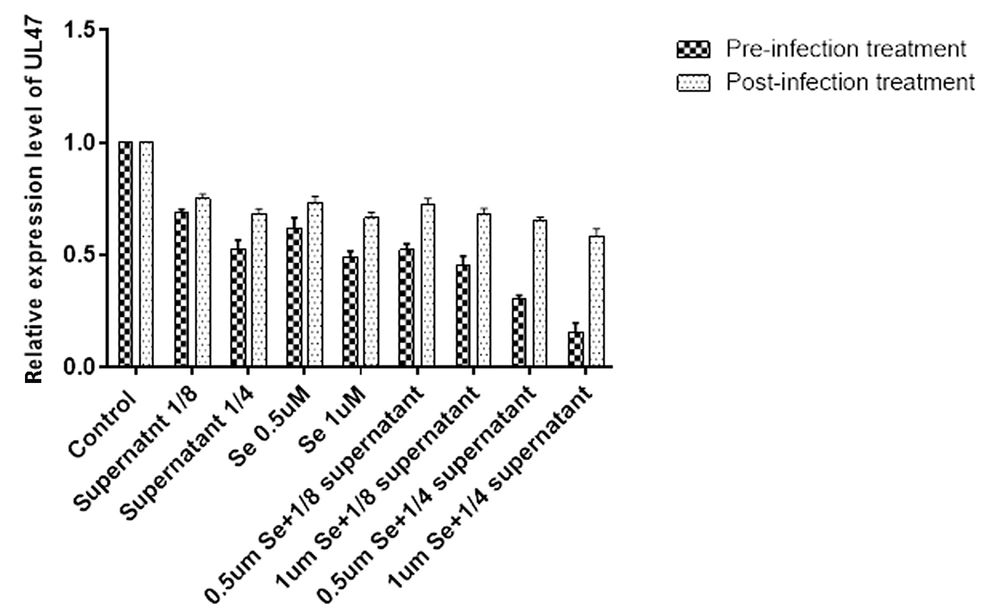
After the incubation of bacterial supernatant (dilutions 1/4 and 1/8) and Se (0.5 and 1 μM) in the pre-infection or post-infection assay, the expression level of the HSV-1 UL47 gene was estimated. The comparison of UL47 expression levels between experiments and control showed similar findings of the viral titration. The expression level of UL47 was lower than the control group in all experiments (Figure 3). The UL47 gene expression levels in post-infection treatments were higher than in pre-infection treatments. Se in concentrations of 0.5 and 1 µM decreased the UL47 gene expression level by about 1.6- and 2-fold, respectively. Similar to viral titer results, the UL47 expression showed a greater decrease when selenium and bacterial supernatant were combined than when only one of the two was used. The combined use of 1 μM Se and 1:4 dilution of B. clausii supernatant caused the greatest drop in the UL47 expression level (6.4-fold) in comparison to other treatment conditions (Figure 3).
5. Discussion
Due to the resistance of HSV-1 to antiviral drugs or some side effects of antiviral compounds, finding alternative antiviral agents is essential (4). The current study aimed to evaluate the effect of selenium and its combination with B. clausii supernatant on the replication of HSV-1. The results showed that both selenium and B. clausii supernatant had a potent inhibitory effect on HSV-1 replication. In addition, the combined inhibitory effect of selenium and B. clausii supernatant on HSV was greater than the individual inhibitory effect of these compounds.
Oxidative stress accompanies some viral infections, such as influenza, parainfluenza, and HSV-1 (16). It is unclear how it influences virus replication. However, the effect of the virus on the antioxidant balance in host cells by the inhibition of antioxidant enzymes, such as glutathione peroxidase, is known (17). Selenium, as a cofactor for glutathione peroxidase, has a key role in antioxidant defense for cells. A deficiency in selenium decreases the activity of glutathione peroxidase. According to a number of studies, a lack of selenium encourages viral replication, mutations, and the formation of more deadly virus types (11). Low glutathione levels are thought to speed up the progression of viral infection either by promoting viral replication or by activating transcription factors, which then result in increased production of inflammatory cytokines, such as interleukin-1 and interleukin-6 (13). The results of the present study indicated that 1 μM of Se can decrease the expression of the HSV-1 UL47 gene and the titer of virus replicated in HeLa cells by about 2.33 log10 TCID50/mL. In this study, the relative expression of the UL47 gene was evaluated because it is representative of virus replication and is involved in enveloping and making a complete virion. Measuring the expression level of UL47 was suitable for confirming the TCID50 results.
Similar results were obtained by Mansour and Salem on BHV-1 (13). They discovered that selenium enhanced glutathione in the pre-infection treatment assay and significantly decreased BHV-1 CPE by 50%. In a study by Verma et al., the infection of Se-deficient Vero cells with West Nile virus leads to enhanced cell death and viral replication (7). In the present study, the inhibitory effect of selenium on HSV-1 replication in pre-infection treatment was greater than in post-infection treatment. Verma et al. previously obtained similar findings (7). This finding reflects the need for time for the synthesis of antioxidant enzymes or might be for receptor rearrangement. This could be because pre-treatment with Se is more antioxidant than post-treatment.
The current study aimed to assess the anti-HSV activity of B. clausii supernatant. The results indicated that the bacterial metabolites had an antiviral effect in both pre-infection and post-infection treatments; however, the pre-treatment assay showed the highest drop in the HSV-1 titer.
One theory is that the B. clausii supernatant interferes with the viral envelope’s ability to attach to the cell surface, inhibiting viral entrance into cells. This conclusion was reached in an earlier study using other probiotic strains (14). Similar to the present investigation, Zabihollahi et al. reported that the vaginal lactobacilli supernatant had a high neutralizing action against HSV-2 in the early stages prior to viral entrance (18). Contrary to the results of the current study, Mousavi et al. showed that Lactobacillus crispatus supernatant had no discernible antiviral action against HSV-2 (19). In a model of rotavirus infection, distinct modes of action of B. clausii strains and their metabolites have recently been identified. In rotavirus-infected cells, pro-inflammatory cytokines, such as interleukin-8 and interferon, were less secreted as a result of the treatment of cells with B. clausii supernatant (7). The results of the present study showed that the combination of selenium and B. clausii supernatant has a stronger anti-HSV effect than when only one of them is used. Selenium might have a synergistic effect on herpes virus proliferation through its antioxidant activity and the supernatant by interfering with virus binding to the cell. However, further studies are needed to clarify the antiviral mechanism of these agents.
5.1. Conclusions
The present data suggest that selenium and the supernatant of B. clausii have potent antiviral activity against HSV-1. The synergistic effects of these two compounds work together to reduce HSV-1 replication. However, further research is required to fully understand how they work to inhibit viruses.