1. Background
Leishmaniasis is an infectious disease prevalent in subtropical and tropical regions. This disease, which manifests various clinical characteristics, is caused by intracellular Leishmania spp. protozoans (1). It is estimated that annually, 20 000 - 30 000 deaths and 700 thousand to one million new cases occur (1-3). The disease appears in several forms (4); the most common is cutaneous leishmaniasis (CL), which poses a significant public health challenge, particularly in the Middle East (5). This infection is often overlooked globally and predominantly affects individuals of lower economic status in both developed and developing countries (6). Leishmaniasis is transmitted when female phlebotomus sandflies bite the skin of a host and deposit promastigotes, which are then phagocytosed by macrophages (7). Macrophages play a crucial role in controlling infections through the production of nitric oxide (NO) (8), which is synthesized by nitric oxide synthase 2 (NOS2) from the substrate L-arginine (9). Numerous studies have demonstrated that immune responses to infectious pathogens are dependent on the expression of NOS2 (8-10).
Additionally, long non-coding RNAs (lncRNAs) significantly influence the polarization of macrophages (11) and have been shown to enhance host immunity and responsiveness to various infections, including those induced by Toxoplasma gondii (12), Schistosoma mansoni (13), and Trichomonas vaginalis (14). Certain lncRNAs play crucial roles in activating macrophages and are key factors in immune responses to infections (15). H19, metastasis-associated lung adenocarcinoma transcript 1 (MALAT), HOX antisense intergenic RNA (HOTAIR), and TNF and HNRNPL Related Immunoregulatory Long Non-Coding RNA (THRIL) are lncRNAs that regulate gene expression in macrophages. They are instrumental in the differentiation, polarization, and activation of macrophages, as well as in the regulation of inflammatory responses (16, 17). Therefore, influencing these lncRNAs may enhance immune responses to infections.
A wide range of drugs is available for treating leishmaniasis, including oral miltefosine, meglumine antimoniate, sodium stibogluconate, paromomycin, and amphotericin B (3, 18-20). However, issues such as extended half-life, toxicity, the development of drug resistance, and teratogenic effects make current therapy options inadequate (21-23). Other factors, including genetic and anthropometric characteristics, geographical area, and particularly immune status, can affect the efficacy of the medications used (24). These challenges have prompted researchers to develop new and innovative treatments to combat this infection. Additionally, ongoing efforts are being made annually to discover new inhibitors for this disease (25).
Melatonin, known as the "darkness hormone," is produced by the pineal gland at night, regulated by the central clock located in the suprachiasmatic nuclei of the hypothalamus. It is also synthesized by immune-competent cells and plays a role in monitoring infections and aiding recovery during acute defense responses (1). The role of melatonin in immune system activities and the regulation of inflammatory processes has been partially characterized; leukocytes are capable of synthesizing and responding to melatonin (26, 27). Previous studies have shown that melatonin is effective in decreasing susceptibility to bacterial infections (28), lethal endotoxemia (29), and several parasitic infections, including those induced by S. mansoni (30), Plasmodium falciparum and P. chabaudi (31), Trypanosoma cruzi (32), L. infantum (33), and L. amazonensis (34). However, various studies have reported conflicting results regarding the regulatory effects of melatonin on NO production (27, 35, 36).
2. Objectives
To the best of our knowledge, no studies have investigated the effect of melatonin on the expression of certain lncRNAs such as H19, MALAT, HOTAIR, and THRIL, as well as NOS activity in L. major-infected macrophages. Therefore, the current study was designed to evaluate the regulatory effect of melatonin on the expression of these lncRNAs and NOS activity in L. major-infected macrophages.
3. Methods
3.1. Parasite Culture
Promastigotes of L. major (MRHO/IR/75/ER) were obtained from the Iranian Biological Resource Center (IBRC), Tehran, Iran. After confirmation via polymerase chain reaction (PCR) technique, the promastigotes were cultured in RPMI 1640 medium containing 100 μg/mL streptomycin, 100 U/mL penicillin, 2 mM L-glutamine, and 10% heat-inactivated Fetal Bovine Serum (FBS) (Gibco, USA). Cultures were maintained at 25°C for one week. In the stationary phase, the promastigotes were carefully transferred to fresh medium at a ratio of 1:4 (6).
3.2. Cell Culture
U937 cell lines, pro-monocytic/human histiocytic lymphoma cells, were acquired from the Pasteur Institute Cell Bank, Tehran, Iran. The cells were maintained in RPMI-1640 medium (Sigma-Aldrich Chemical Co, St. Louis, MO, USA) supplemented with 100 μg/mL streptomycin, 100 U/mL penicillin, 2 mM L-glutamine, and 10% heat-inactivated FBS (Gibco, USA), and incubated at 37°C in a 5% CO2 atmosphere. The cells were stimulated with phorbol myristic acid (PMA) for three days to differentiate the monocytes into macrophages. Upon reaching approximately 80% confluency, the cells were passaged (6, 37).
3.3. In Vitro Macrophage Infection
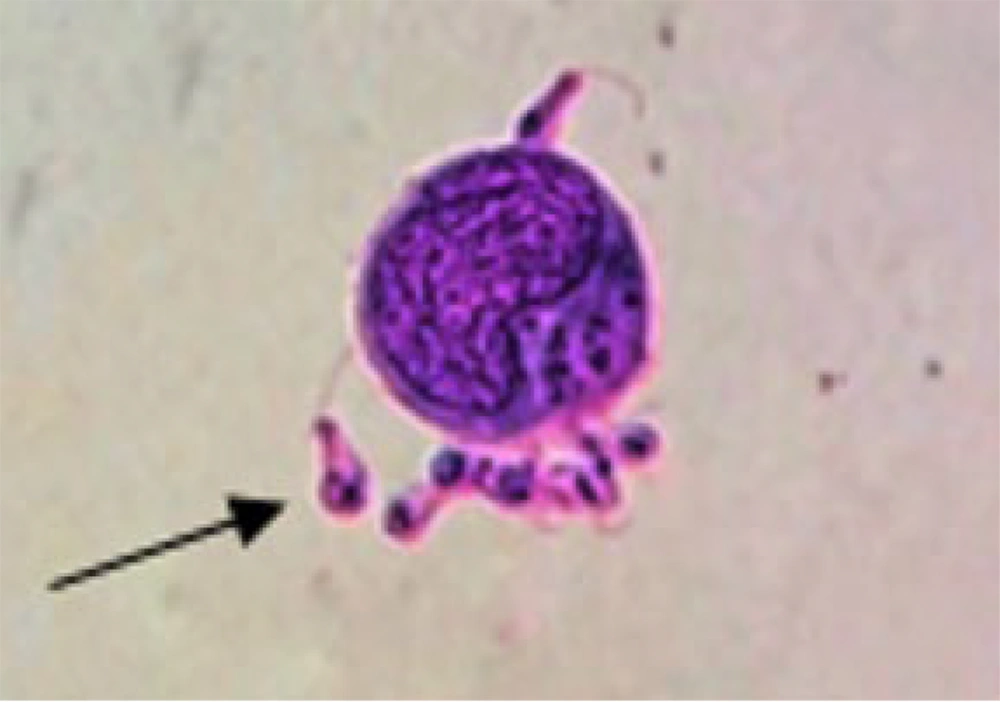
Macrophage infection was carried out as previously described (6). Briefly, 1 × 106 cells/mL were seeded in a 6-well plate and incubated at 37°C for 24 hours. Subsequently, the macrophages were infected with promastigotes and maintained at 37°C for 4 hours. Non-phagocytosed promastigotes were removed by washing with Phosphate-Buffered Saline (PBS). After the PBS wash, the cells were treated with melatonin (98% purity, CAS no. 73-31-4, Sigma, USA) at concentrations of 3, 10, 30, and 100 nM for durations of 4, 24, and 48 hours. Melatonin was dissolved in Dimethyl sulfoxide (DMSO). This setup included a treated group (Leishmania-infected macrophages treated with melatonin) and a control group (Leishmania-infected macrophages treated with DMSO). Finally, all cells were harvested using PBS containing 0.6 mM Ethylenediaminetetraacetic acid (EDTA) and stored at -80°C until further analysis. For confirmation of Leishmania infection, samples were smeared on slides, air-dried, fixed with methanol, and stained using the Giemsa method. All slides were carefully examined under a light microscope at a magnification of 1000x (38).
3.4. RNA Extraction, Reverse Transcription, and Quantitative PCR for lncRNAs
Initially, RNA samples were isolated using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quantity and quality of the RNA samples were assessed using a NanoDrop Spectrophotometer (Thermo Scientific, USA) and gel electrophoresis. Table 1 lists the sequences of all primers used. Subsequently, cDNA synthesis of lncRNAs was performed using a cDNA synthesis kit (TaKaRa Bio, Tokyo, Japan) in a total volume of 20 μL. The synthesized cDNAs were immediately stored at -80°C for use in quantitative PCR (qPCR). The relative expression of lncRNAs H19, MALAT, HOTAIR, and THRIL was determined by qPCR using specific primers and Real Q Plus 2X Master Mix Green High ROX™ (Amplicon, Denmark). Reactions were conducted on a StepOne Plus™ instrument (ABI, USA) in a final volume of 20 μL, containing 1 μL of cDNA, 10 μM of each primer pair, 2X SYBR Green PCR Master Mix, and RNase-free water. The expression of lncRNAs was normalized to GAPDH expression as the internal control, and the delta-delta CT protocol was used to calculate the relative expression. All assays were performed in triplicate.
| Gene and Forward/ReversePrimers | Sequence (5' - > 3') | Product Length | Tm |
| MALAT1 | 179 | ||
| Forward | GGATTCCAGGAAGGAGCGAG | 59.89 | |
| Reverse | ACATATTGCCGACCTCACGG | 60.18 | |
| H19 | 119 | ||
| Forward | CGGCCTTCCTGAACACCTTAG | 60.68 | |
| Reverse | ATGTTGTGGGTTCTGGGAGC | 60.25 | |
| HOTAIR | 148 | ||
| Forward | TCTAACTGGCAGCACAGAGC | 60.04 | |
| Reverse | CAAAGTCCCGTTTGCAGCAG | 60.32 | |
| THRIL | 146 | ||
| Forward | TCCAAACTGAGACCTGGACC | 58.94 | |
| Reverse | CTGGGGATCACGACTGTCTC | 59.54 | |
| GAPDH | 100 | ||
| Forward | CAGCCTCAAGATCATCAGCAATG | 60.0 | |
| Reverse | CATGAGTCCTTCCACGATACCA | 59.57 |
Abbreviation: Tm, melting temperature.
3.5. Quantification of NOS Activity
The nitrite (NO2-) content, indicative of NO production, was measured in the supernatant cultures of Leishmania-infected macrophages using the Griess reaction, as previously described (39). As a standard, the absorbance was measured at 540 nm following the incubation of 50 μL of the solution containing N-[naphthyl] ethylenediamine dihydrochloride (1 mg/mL), 5% phosphoric acid, and sulfanilamide (10 mg/mL) with 50 μL of the supernatant (39).
3.6. Statistical Analysis
The Shapiro-Wilk test was applied to assess the normality of data. Differences among groups were evaluated using one-way ANOVA with Tukey's HSD post hoc test for normally distributed variables and the Kruskal-Wallis H test for non-normally distributed variables. Data for normally distributed variables were presented as mean ± standard error of the mean (SEM), and non-normally distributed data were presented as the median and interquartile range (IQR). All analyses were performed using IBM SPSS AMOS 21.0 (SPSS, Inc., Chicago, IL) and GraphPad Prism software, version 8 (GraphPad Software, CA). A P-value < 0.05 was considered statistically significant.
4. Results
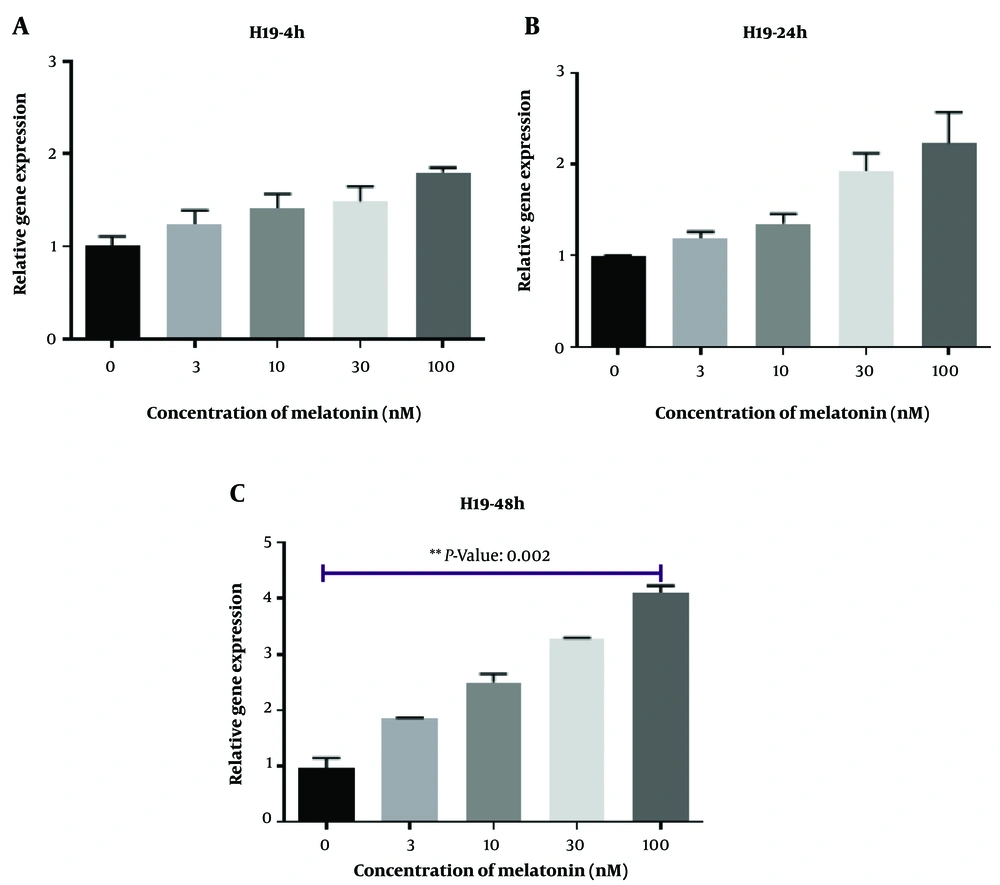
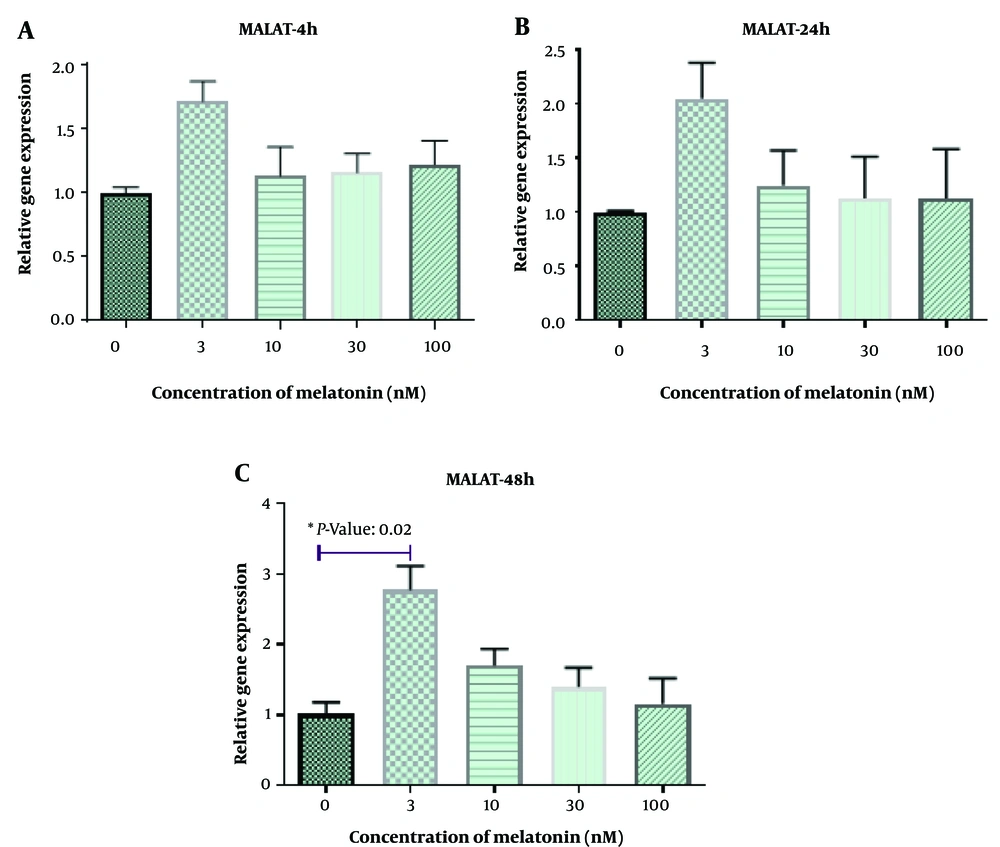
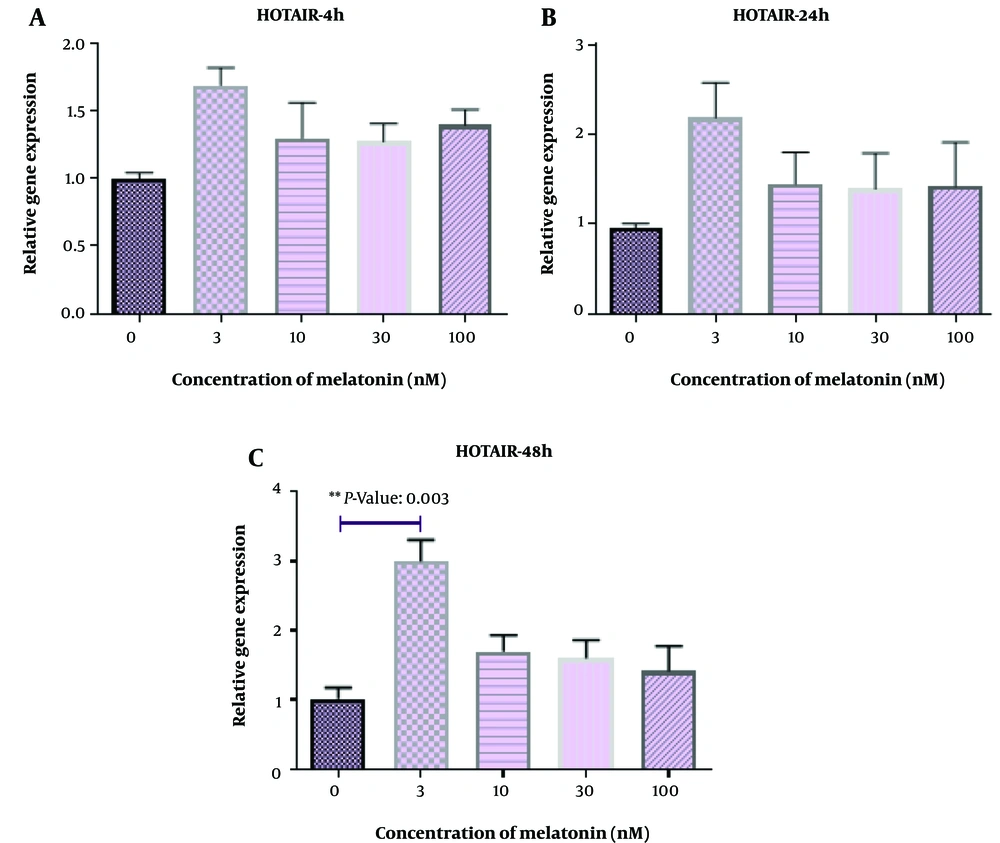
Figure 1 displays L. major-infected macrophages stained using the Giemsa method. Furthermore, Figures 2 - 6 illustrate the effects of various concentrations of melatonin on the gene expression of H19, MALAT, HOTAIR, THRIL, and NOS activity in L. major-infected macrophages after treatments of 4, 24, and 48 hours, respectively. Our findings indicate that the expression of H19 increased approximately fourfold in the group treated with melatonin at a dose of 100 nM compared to the control group after 48 hours (Figure 2 P = 0.002). However, no significant differences were observed among other groups compared to the control group (P > 0.05). According to Figures 3C and 4C, a significant up-regulation in the expression of MALAT and HOTAIR was noted in L. major-infected macrophages treated with a concentration of 3 nM of melatonin compared to the control group after 48 hours of incubation (P = 0.02 and P = 0.003, respectively). Nevertheless, there were no significant differences in the expression of MALAT and HOTAIR among other groups at different concentrations of melatonin (P > 0.05).
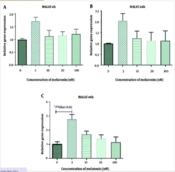
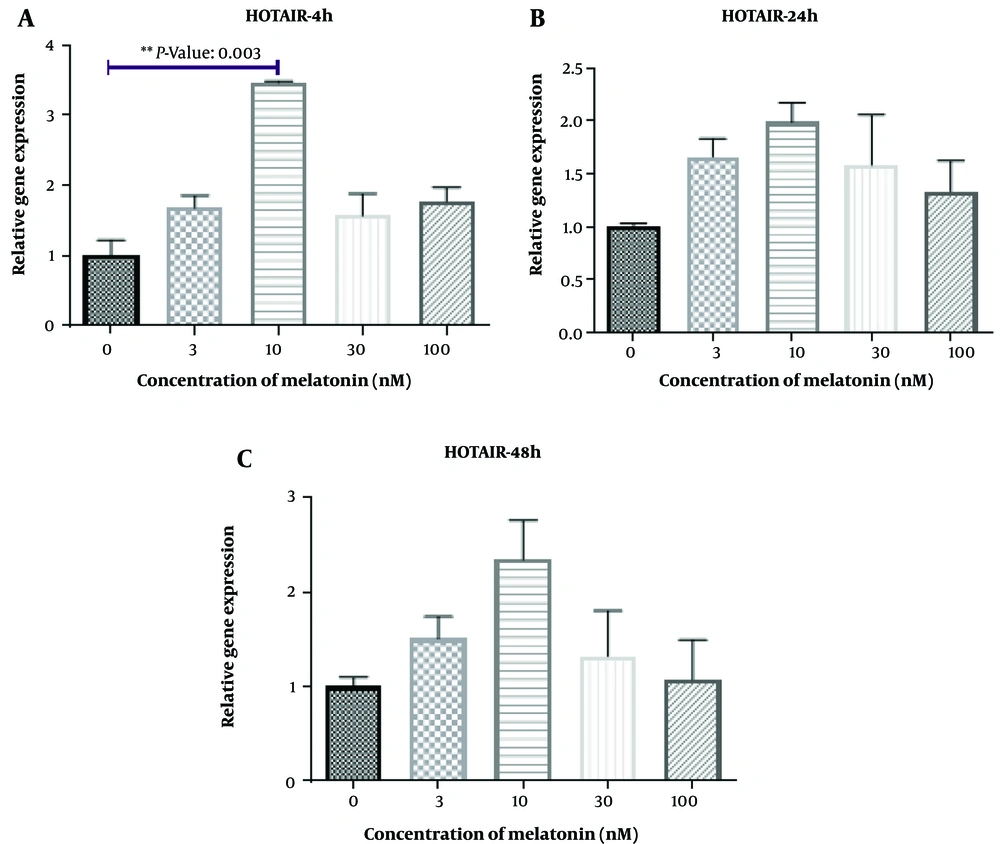
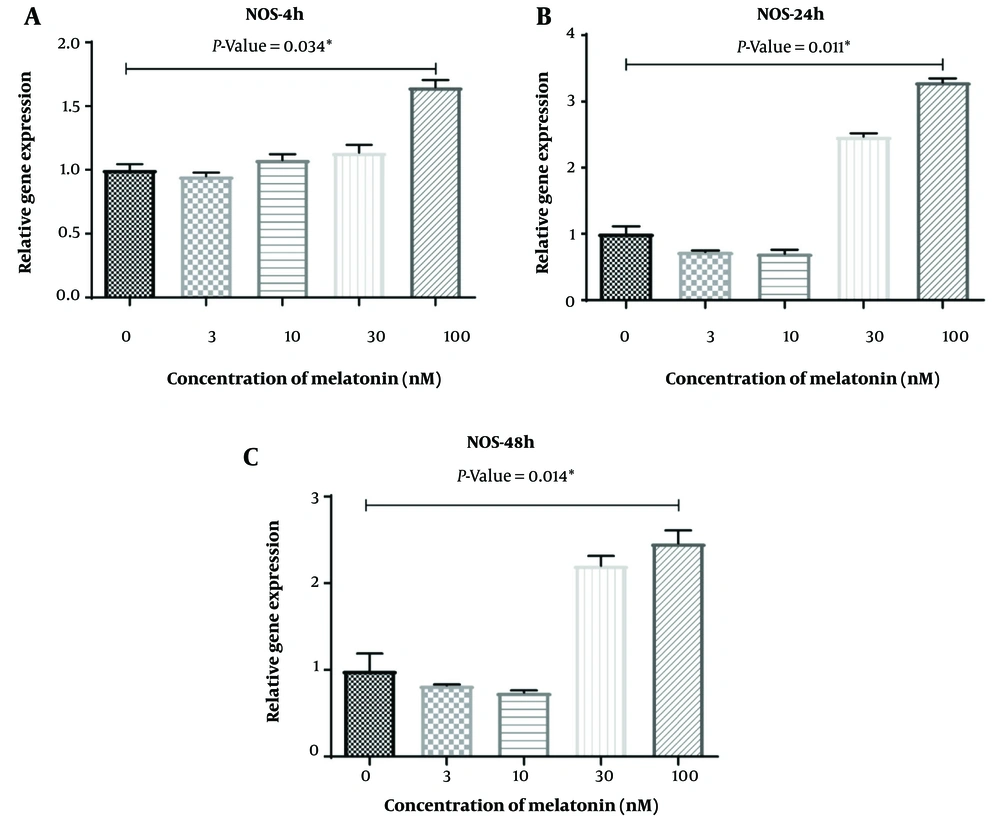
Regarding the expression of THRIL, a significant difference was observed in macrophages treated with a concentration of 10 nM of melatonin compared to the control group after 4 hours of treatment (P = 0.003). However, there were no significant differences among other groups compared to the control group at different concentrations of melatonin (P > 0.05). Additionally, as shown in Figure 6A and C, an increase in NOS activity was detected in L. major-infected macrophages treated with melatonin at a concentration of 100 nM compared to the DMSO group after 4 hours, 24 hours, and 48 hours of treatment (P = 0.034, P = 0.011, and P = 0.014, respectively). Yet, no significant differences were observed among other groups compared to the control group at different concentrations of melatonin (P > 0.05).
5. Discussion
Leishmania protozoa are responsible for causing leishmaniasis, which manifests a range of symptoms including visceral, mucocutaneous, cutaneous, and disseminated diseases (23). T helper-1 cells (Th1) secrete cytokines such as interferon-gamma (IFN-γ) and interleukin 12 (IL-12), which lead to immunity and resistance to leishmaniasis. Conversely, Th2 cells mediate susceptibility to infection, exacerbating the disease through the production of IL-4, IL-5, and IL-10 (6). Leishmania major employs various strategies to evade detection by the immune system, forming a symbiotic relationship with its host to ensure its survival within macrophages (9). Meanwhile, melatonin stimulates antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase, and inhibits lipoxygenase (40). Additionally, leukocytes can synthesize and respond to melatonin (26). Indeed, melatonin has been shown to decrease susceptibility to bacterial (28) and parasitic infections (30). Given the immunomodulatory properties of melatonin and the key role of some lncRNAs in modulating the immune system, this study was designed to evaluate the regulatory effect of melatonin on the expression of lncRNAs and NOS activity in L. major-infected macrophages.
We observed an upregulation in the expression of MALAT and HOTAIR in L. major-infected macrophages treated with melatonin (3 nM) compared to the control group after 48 hours of treatment. HOTAIR plays a crucial role in activating NF-κB (an inducible transcription factor involved in regulating inflammation) in macrophages and has been identified as a potential factor in immune responses to infections (15). It is known that Leishmania spp. parasites can inhibit NF-κB activity in macrophages infected with promastigotes (39). Thus, the enhancing effect of melatonin on this specific lncRNA may improve the ability of macrophages to combat L. major infection. Conversely, it has been reported that MALAT1 interacts with the NF-κB factor, suppressing its activity and potentially reducing the production of inflammatory cytokines such as IL-6 and TNF-α. These contradictory findings suggest that the regulatory effects of these lncRNAs on the immune system are complex, and maintaining a balance between them (HOTAIR and MALAT1) is crucial (41).
Additionally, the results of this study show that the expression of H19 was significantly increased in the group treated with melatonin (100 nM) compared to the control group after 48 hours of incubation. However, no significant differences were observed among the other groups compared to the control group. To the best of our knowledge, this is the first study to evaluate specific lncRNAs in L. major-infected macrophages. H19, one of the earliest detected lncRNAs, primarily resides in the cytoplasm and is located on chromosome 11p15.5 in humans; it functions as a riboregulator or regulatory RNA (42). Despite this, findings regarding H19 are conflicting. This lncRNA plays key roles in the inflammatory process, fibrosis, and some cancers (43-45). Interestingly, H19 enhances M2 polarization (43) and has been shown to act as a molecular sponge for let-7 (46). Moreover, in 2018, Hashemi et al. (47) demonstrated that inhibition of let-7a in macrophages could represent a novel approach to treating leishmaniasis.
As previously mentioned, macrophages control infections using NO produced by NOS2 (9). In contrast, Arginase 1 (ARG1) is an immune-regulating enzyme that can reduce NO production by macrophages (48, 49). Th1-related cytokines such as IFN-γ, TNF, and GM-CSF activate phenotype 1 macrophages by increasing NOS2 expression and decreasing ARG1 levels, thus controlling the proliferation of Leishmania spp. parasites (50, 51). In accordance with these findings, our study observed an increase in NOS activity in the melatonin group at a concentration of 100 nM compared to the DMSO/control group after 4 hours, 24 hours, and 48 hours of treatment. However, previous studies have shown that melatonin has varying regulatory effects, both increasing (35) and decreasing (34), on No production (34, 35).
Furthermore, the expression of THRIL was significantly increased in the melatonin group (10 nM) compared to the control group after 4 hours of treatment. Notably, THRIL is involved in the regulatory expression of TNF-α in human monocytes (52, 53). TNF-α is known as a factor that activates macrophages to kill the Leishmania spp. parasite and plays an important role in protective immunity in cutaneous leishmaniasis (54). Therefore, the increase of THRIL mediated by melatonin could potentially decrease this infection in L. major-infected macrophages. However, further complementary studies are required in this area. The limitations of the present study include the lack of evaluation of other species related to leishmaniasis, and other lncRNAs and proteins associated with inflammation. Future research is necessary to confirm these findings and to understand their mechanisms. Our findings demonstrated that melatonin could increase the expression of H19 at a dose of 100 nM (after 48 hours), THRIL at a dose of 10 nM (after 4 hours), MALAT and HOTAIR at a dose of 3 nM (after 48 hours), as well as NOS activity at a dose of 100 nM (after 4, 24, and 48 hours) in L. major-infected macrophages. The changes occurred in a dose-time dependent manner. More complementary studies are needed in this regard.
5.1. Conclusions
To the best of our knowledge, no studies have investigated the effect of melatonin on the expression of lncRNAs such as H19, MALAT, HOTAIR, and THRIL, as well as NOS activity in L. major-infected macrophages. Our findings indicate that melatonin could increase the expression of H19, THRIL, MALAT, and HOTAIR, as well as NOS activity in Leishmania major-infected macrophages. The enhancing effect of melatonin on these lncRNAs may improve the potential of macrophages to combat L. major infection. Future studies should consider evaluating the inhibition of lncRNAs and NOS mRNA expression levels through inhibitory transfection experiments to confirm the results of the current study.