1. Background
Bloodstream infections (BSIs) contribute to significant morbidity and mortality worldwide. Rapid identification of pathogens from blood culture samples is crucial for initiating timely and appropriate antimicrobial therapy (1-3). Blood culture remains the reference method for detecting bacterial infections in the bloodstream, in addition to clinical evaluation. Although commercial blood culture systems have reduced the time required for pathogen detection, confirming BSIs still depends on traditional methods, such as Gram staining, culturing on solid media, biochemical identification, and antimicrobial susceptibility testing. Traditional culture-based identification methods require 24 - 72 hours for pathogen detection, potentially delaying treatment. Each hour of delay in initiating appropriate antimicrobial therapy in septic patients increases the mortality rate by 7.6% (4, 5). Therefore, rapid identification of the microorganisms responsible for bacteremia is essential for starting the appropriate therapy.
Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) has revolutionized microbial identification by providing rapid and accurate results. In recent years, commercial and in-house MALDI-TOF-MS procedures have been developed for the detection of microorganisms directly from the blood culture bottle (BCB) to reduce turnaround time. However, the presence of blood components and other interfering substances in direct blood culture samples can complicate pathogen identification. Various manual and commercial protocols involving centrifugation, washing, and protein extraction have been proposed to address these challenges. This study evaluates a simplified in-house protocol to improve direct pathogen identification efficiency from blood cultures (6-10). Most protocols are often time-consuming, require specific equipment, and are laborious for routine use. Therefore, there is a need to develop simple, easy-to-use, and reliable methods for direct bacterial identification from blood culture.
2. Objectives
The purpose of this research was to assess the effectiveness of an easy, quick, and affordable in-house method for detecting microorganisms directly from BCBs with the VITEK MS system.
3. Methods
3.1. Sample Collection
Blood samples collected at Marmara University Pendik Training and Research Hospital were inoculated into BacT/ALERT BCBs (aerobic, anaerobic, and pediatric). Only BCBs with positive results were included in the study. A total of 296 samples exhibiting monomicrobial growth were analyzed.
3.2. Blood Culture Processing
All BCB (aerobic, anaerobic, and pediatric) were incubated at 37°C in an automated BacT/ALERT system for up to 5 days until reported positive. Each positive BCB was Gram stained using an automated Gram staining system and inoculated onto various agar plates, including 5% sheep blood agar, chocolate agar, and MacConkey agar. We excluded samples that did not grow on aerobic culture plates, such as anaerobic bacteria. After overnight incubation, identification of bacteria grown on agar plates was done using VITEK MS.
3.3. In-house Extraction Method from Blood Culture
1. Four milliliters of blood culture broth were centrifuged at 2000 g for 30 seconds to separate the blood cells.
2. The supernatant was then subjected to a second centrifugation at 15,500 g for 5 minutes to collect bacterial cells.
3. The bacterial pellet was washed three times with deionized water to remove contaminants.
4. The final pellet was resuspended in 300 µL of water and mixed with 900 µL of absolute ethanol.
5. Following centrifugation at 15,500 g for 2 minutes, the supernatant was discarded.
6. The pellet was re-suspended in a 50 µL solution of 70% formic acid and 50 µL of acetonitrile.
7. After the final centrifugation step at 15,500 g for 2 minutes, 1 µL of the supernatant was transferred onto a VITEK MS plate, air-dried, and analyzed using MALDI-TOF-MS.
3.4. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry
Each spot was overlaid with 0.5 µL of formic acid and 1 µL of the alpha-cyano-4-hydroxycinnamic acid (HCCA) matrix solution. After allowing it to air dry at room temperature, the VITEK MS plate was analyzed using the VITEK MS IVD system. The protein profile for each sample, covering an m/z range from 3,000 to 15,000, was obtained from 100 measurements. Results with confidence levels ranging from 90% to 98% were considered reliable for species and genus identification, while results with confidence below 90% were deemed unacceptable.
Data Analysis: Results from MALDI-TOF-MS were compared to conventional culture-based identification results and evaluated in three categories: Concordant (species/genus match), discordant (genus-level mismatch), and unidentified.
1. Concordant: The concordance of identification results obtained from agar plates and direct BCBs at the species and genus level.
2. Discordant: The discordance of identification results obtained from agar plates and direct BCBs at the genus level.
3. Unidentified: The concordance of identification results obtained from agar plates and direct BCBs at the species and genus level.
Finally, chi-square analysis was used to assess the statistical significance of differences in identification accuracy between groups. A P-value < 0.05 was considered statistically significant.
4. Results
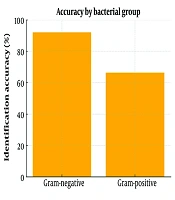
Among the 296 positive blood cultures examined, Gram-negative bacteria were correctly identified in 92.0% of instances, whereas the identification of Gram-positive bacteria had an accuracy of 66.4% (Figure 1). Chi-square analysis indicated this difference was statistically significant (P < 0.01). Gram-negative bacteria exhibited high concordance with standard culture methods, while Gram-positive bacteria showed some discrepancies.
A total of 134 Gram-positive microorganisms were identified in blood culture samples prepared using the conventional culture method. Of these, 89 (66.4%) were accurately identified at the species and genus level using an in-house extraction technique from BCBs. Discordant results were obtained in 7 strains, and these were at the genus level. On the other hand, 38 strains were unidentified. Errors in identification were mostly observed with Staphylococcus aureus (14/41), S. epidermidis (9/32), S. haemolyticus (4/7), and Enterococcus faecium (6/12) (Table 1).
| Organisms | No. of Strains | No. of Concordant | No. of Discordant | No. of Unidentified |
|---|---|---|---|---|
| Gram negative microorganisms | 162 | 149 (92.0) | 0 | 13 (8.0) |
| Enterobacteriaceae | ||||
| Escherichia coli | 65 | 60 | - | 5 |
| Klebsiella pneumonia | 42 | 40 | - | 2 |
| Enterobacter cloacae | 6 | 5 | - | 1 |
| Serratia marcescens | 3 | 3 | - | - |
| E. aerogenes | 2 | 2 | - | - |
| K. oxytoca | 1 | 1 | - | - |
| Proteus mirabilis | 1 | - | - | 1 |
| Other | ||||
| Acinetobacter baumannii | 26 | 25 | - | 1 |
| Pseudomonas aeruginosa | 11 | 9 | - | 2 |
| Stenotrophomonas maltophilia | 2 | 1 | - | 1 |
| Aeromonas hydrophila/caviae | 1 | 1 | - | - |
| Burkholderia cepacia | 1 | 1 | - | - |
| Moraxella catarrhalis | 1 | 1 | - | - |
| Gram positive microorganisms | 134 | 89 (66.4) | 7 (5.2) | 38 (28.4) |
| Staphylococcus spp. | ||||
| Staphylococcus aureus | 41 | 27 | 3 | 11 |
| S. epidermidis | 32 | 23 | - | 9 |
| S. hominis | 14 | 10 | - | 4 |
| S.haemolyticus | 7 | 3 | 1 | 3 |
| S. lugdunensis | 3 | 3 | - | - |
| S. capitis | 2 | 2 | - | - |
| S. caprae | 1 | 1 | - | - |
| S. simulans | 1 | - | 1 | - |
| S. warneri | 2 | 1 | - | 1 |
| Streptococcus spp. | ||||
| Streptococcus pneumonia | 4 | 3 | - | 1 |
| S. pyogenes | 2 | - | 1 | 1 |
| S. dysgalactiae | 1 | 1 | - | - |
| S. parasanguinis | 1 | 1 | - | - |
| S. sanguinis | 1 | 1 | - | - |
| S. anginosus | 1 | - | - | 1 |
| Enterococcus spp. | ||||
| Enterococcus faecium | 12 | 6 | 1 | 5 |
| E. faecalis | 9 | 7 | - | 2 |
| Total | 296 | 238 (80.4) | 7 (2.4) | 51 (17.2) |
a Values are expressed as No. (%).
A total of 162 Gram-negative microorganisms were identified in the blood culture samples prepared using the traditional culture method. Out of these, 149 (92.0%) were accurately identified at the species and genus level through an in-house extraction technique from BCBs. While no discordant results were found for Gram-negative microorganisms compared to the conventional culture method, 13 strains were not identified by the in-house method (Table 1).
5. Discussion
The main task of a clinical microbiology laboratory is to correctly identify pathogens that cause infectious diseases in a short time and to assist clinicians in the implementation of appropriate treatment protocols by determining antibiotic susceptibility profiles. Blood culture is considered the gold standard for diagnosing BSIs. Rapid and accurate pathogen identification is crucial for effective antimicrobial treatment (11). The average time for positive signaling of blood culture systems is approximately 24 hours. Gram staining and subculture processes also take at least 24 hours. Therefore, at least 48 hours are required for the identification of pathogens that reproduce in blood culture by traditional methods.
In the last decade, several rapid methods (real-time PCR, multiplex PCR, fluorescent in situ hybridization, and peptide nucleic acid hybridization) have come into use for the rapid identification of pathogens. However, not all pathogens can be detected with these methods, and the equipment and reagents required for the method are quite expensive (12). Over the last ten years, MALDI-TOF-MS has become a widely used tool for the rapid identification of microorganisms cultured on solid media. The MALDI-TOF-MS has had a revolutionary effect in microbiology laboratories due to its rapid and high-throughput detection of various types of pathogens (13).
Recently, direct identification protocols from positive BCBs have been developed to reduce the time of diagnosis. Several investigations have examined the use of MALDI-TOF-MS for directly identifying microorganisms in BCBs, employing different protocols (6-8). Consequently, it is aimed to better analyze the bacterial proteome by performing bacterial protein extraction through pre-processing with in-house and commercial protocols developed before the BCB is analyzed. In this research, we assessed the effectiveness of MALDI-TOF-MS for processing blood cultures using an in-house direct protocol prior to analysis.
In our study, we used a simple method made with equipment that can be easily found in every laboratory. With the use of such simple diagnostic protocols in clinical microbiology laboratories, rapid results can be produced that can positively affect the prognosis. In this study, we found that Gram-negative bacteria were identified more accurately with MALDI-TOF-MS compared to Gram-positive bacteria (92.0% versus 66.4%). These results are similar to other studies with MALDI-TOF-MS in the literature (9, 14, 15). For example, the identification rate was 85.0% for Gram-negative aerobes, with Gram-positive aerobes following (78.2%) in Lin et al.'s study (9). In the study by Jo et al., the overall correct identification rate was 81.8% (208/254), with a success rate of 73.9% for Gram-positive isolates and 92.6% for Gram-negative isolates (14). Mestas et al. found that organisms were correctly identified to the species level, with a significantly higher identification rate for Gram-negative organisms (90.3%) compared to Gram-positive organisms (78.4%) (15).
These results are supported by previous studies on direct MALDI-TOF MS from positive blood cultures. Tsuchida et al. achieved 85.5% overall accuracy and 76.1% for Gram-positive organisms with an optimized in-house lysis-filtration method (16). A large-scale study on 538 samples demonstrated a 93.4% accuracy for Gram-negative and 78.9% for Gram-positive bacteria (17). These findings corroborate our observations and highlight the potential of direct workflows while also confirming limitations in Gram-positive detection.
The correct identification of Gram-negative bacteria has a significant impact on the choice of antimicrobial agent to be used in treatment because there are many antibiotics that can be used to treat Gram-negative bacteria, and resistance to antimicrobials is higher. The in-house method demonstrated high accuracy for Gram-negative bacteria, consistent with findings from previous studies. The lower identification rate for Gram-positive bacteria may be attributed to the complex cell wall structure, lower bacterial concentration, or interference from blood components.
Optimization strategies, including chemical agents such as saponin and SDS, may enhance identification rates. However, similarly, the correct identification rates of Gram-positive bacteria were lower than those of Gram-negative bacteria in these studies (10, 18-23). In our study, 17.2% of the isolates were not identified by the method used. Of these, 54.9% were Staphylococcus species, consisting of 11 S. aureus, 9 S. epidermidis, 4 S. hominis, 3 S. haemolyticus, and 1 S. warneri. The rate of unidentified isolates has been reported to be between 10.0% and 13.0% in other studies (8, 14, 24). While discordant results were not detected in the Gram-negative bacteria, they were observed in seven Gram-positive bacteria. The rates of discordant results in other studies ranged from 0% to 4%, and it was found to be 2.4% in our study. Most of these results were Gram-positive bacteria, commonly Staphylococci species, as seen in other studies (14, 24, 25).
The identification of bacteria causing BSI was accomplished in a short time, like 1 hour, with the method used in our study. The short identification period allows the treatment of patients with BSI to be started in a short time. The advantage of this method is that it provides results 48 hours earlier than the traditional identification method (26). It is also a simple and cost-effective method. Early detection of pathogens causing BSI can significantly reduce mortality rates, especially in critically ill patients, through early and effective treatment (27). At the same time, if these methods can be used to detect antimicrobial resistance, more accurate treatment protocols specific to the pathogen can be determined. In this way, misuse and overuse of broad-spectrum antibiotics are prevented (28).
5.1. Conclusions
In conclusion, the in-house method offers a rapid, cost-effective, and practical alternative for direct microbial identification from blood cultures. While Gram-negative bacteria were accurately identified, further improvements are needed to enhance the accuracy for Gram-positive bacteria. Future research should focus on refining lysis and extraction techniques tailored for Gram-positive organisms and integrating the detection of antimicrobial resistance markers directly from blood culture samples. Such advances could support more targeted therapy, reduce hospital stay durations, and limit the emergence of resistance due to inappropriate antimicrobial use.