1. Background
Achromobacter species is a motile, aerobic, oxidase-positive, non-fermentative Gram-negative bacillus (1). This microorganism is widely distributed in the environment and is most frequently associated with healthcare-associated infections (1, 2). The majority of cases occur in patients with some form of immunosuppression, particularly those with hematologic malignancies, with primary bacteremia being the most prevalent clinical presentation (3-6). Additional documented infections include urinary tract infections, abscesses, osteomyelitis, corneal ulcers, prosthetic valve endocarditis, peritonitis, and pneumonia (7-10). Surgical site infections caused by Achromobacter spp. are increasingly reported in the literature (11). In immunocompromised hosts, it is known to cause opportunistic infections, including bloodstream infections, pneumonia, and urinary tract infections (12).
The species is ubiquitous in aquatic environments and is capable of colonizing various aqueous solutions, such as dialysis fluids, ultrasound gels, and even disinfectants used in hospital settings (13, 14). Contaminated hospital environments, particularly water-containing equipment and healthcare workers’ hands or gowns, can serve as reservoirs for these pathogens and play a critical role in the transmission of nosocomial infections (15). Resistance to beta-lactams, fluoroquinolones, and aminoglycosides, which are frequently used in empirical treatment, has been increasing in Achromobacter spp. isolates (16). The production of various beta-lactamase enzymes and the activity of efflux pumps play a significant role in the development of this resistance (1).
2. Objectives
The aim of this study was to investigate the resistance profile and microbiological characteristics of Achromobacter spp. isolated in a tertiary health institution during a 10-year period. It also aimed to examine the demographic data, treatment protocols, treatment responses, and prognoses of patients in whom Achromobacter spp. was isolated in any sample.
3. Methods
3.1. Study Design
A retrospective study was conducted from March 2014 to March 2024, utilizing original data extracted from the Bacteriology Laboratory's database, which cataloged all clinical samples positive for Achromobacter spp. The study was performed at a 1300-bed University Hospital.
3.2. Study Population and Data Sources
The study included all patients from whom Achromobacter spp. were isolated in clinical specimens during the specified time frame. Duplicate isolates, defined as strains with identical antimicrobial susceptibility profiles isolated from the same sample type in the same patient, were excluded. Isolates obtained from a single positive culture without significant clinical indicators were considered as colonization and excluded from the study. Details regarding the patients' age, gender, immunosuppression status, co-morbidities, and treatment unit were obtained from patient files. Information on antibiotic regimens and 28-day clinical outcomes was recorded. Immunocompromised status was ascertained based on the following parameters: A prior history of organ transplantation, chemotherapy, radiotherapy, or immunosuppressive therapy, as well as the presence of co-morbidities requiring sustained corticosteroid administration. Polymicrobial infection was delineated as the concurrent isolation of more than one microorganism from a given specimen type.
3.3. Antimicrobial Susceptibility Testing
Achromobacter spp. growth in clinical samples brought to the Bacteriology Unit of the Central Laboratory of Erciyes University Faculty of Medicine between March 2014 and March 2024 was retrospectively examined. The hospital information management system was used during the examinations. In the identification of bacteria, in addition to phenotypic properties such as colony morphology, pigment production, odor of bacteria, and gram staining patterns, biochemical tests such as catalase, oxidase, urease, indole, motility, esculin hydrolysis, ornithine, lysine, arginine decarboxylation, and oxidation-fermentation tests for various sugars such as sucrose, xylose, and glucose were used. In addition to these tests, Vitek 2 (Biomeriux, France) and Phoenix M50 (Becton Dickinson, USA) automated systems were used between 2014 and March 2020, and BD Sirius (Bruker, Germany) and Vitek MS Prime (Biomeriux, France) MALDI TOF MS systems were used to improve species-level identification between 2020 and March 2024.
Disk diffusion test and gradient strip tests were used in antibiotic susceptibility tests of the isolates (ceftazidime, cefepime, ciprofloxacin, piperacillin-tazobactam, meropenem, and trimethoprim-sulfamethoxazole). In the evaluation of antibiotic susceptibility tests, CLSI M100 and M45 documents were used between 2014 and 2020, and European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations were used after Achromobacterxylosoxidans was added to the EUCAST clinical breakpoint tables after 2020. Identification of subgroups could not be made because some isolates could not be revived.
3.4. Treatment Description
The use of appropriate antibiotic therapy was deemed sufficient if at least one drug from the tested antimicrobial panel was employed in the patient’s treatment plan.
3.5. Statistical Analysis
Data was analyzed using the SPSS 27.0 statistical software package. Patients' demographic characteristics, treatment departments, antimicrobial resistance patterns, and clinical outcomes were compared based on the presence of bacteremia and polymicrobial infections. The chi-square or Fisher's exact test was employed for the analysis of categorical variables, while the Shapiro-Wilk test was utilized to assess the normality of continuous variables. For variables demonstrating normal distribution, an independent t-test for two samples was applied, whereas the Mann-Whitney U test was used for those not following a normal distribution. A P-value of less than 0.05 was considered statistically significant.
4. Results
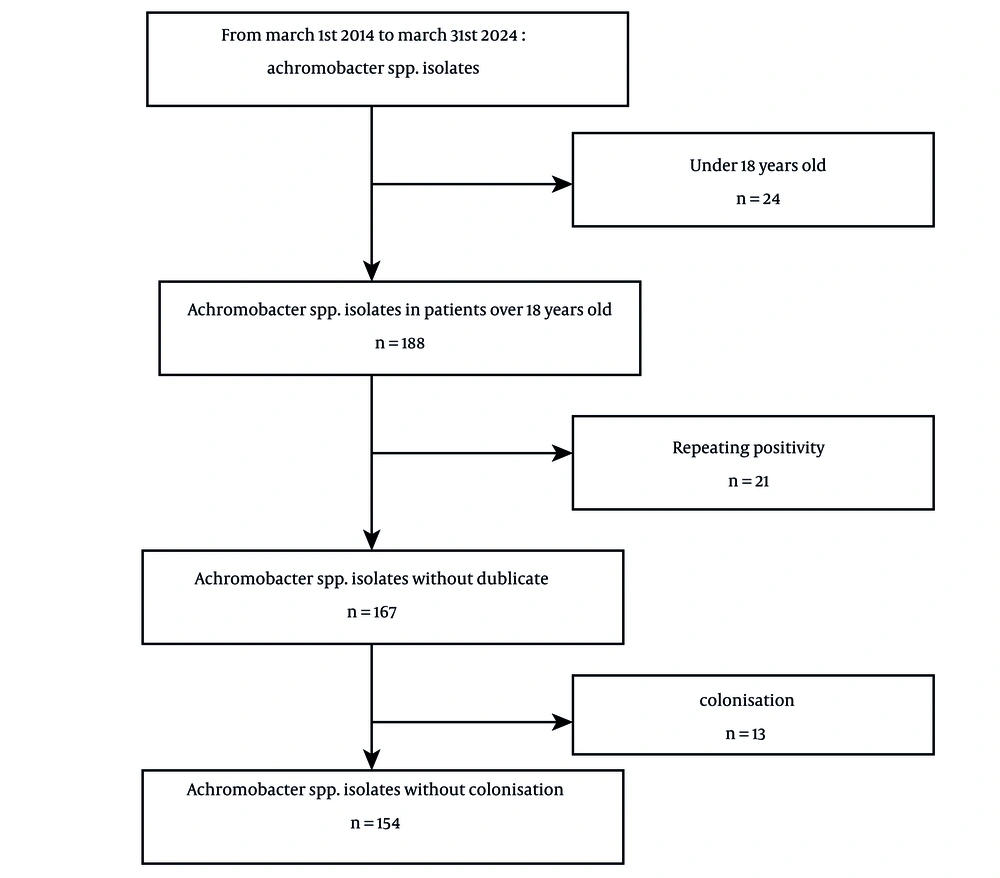
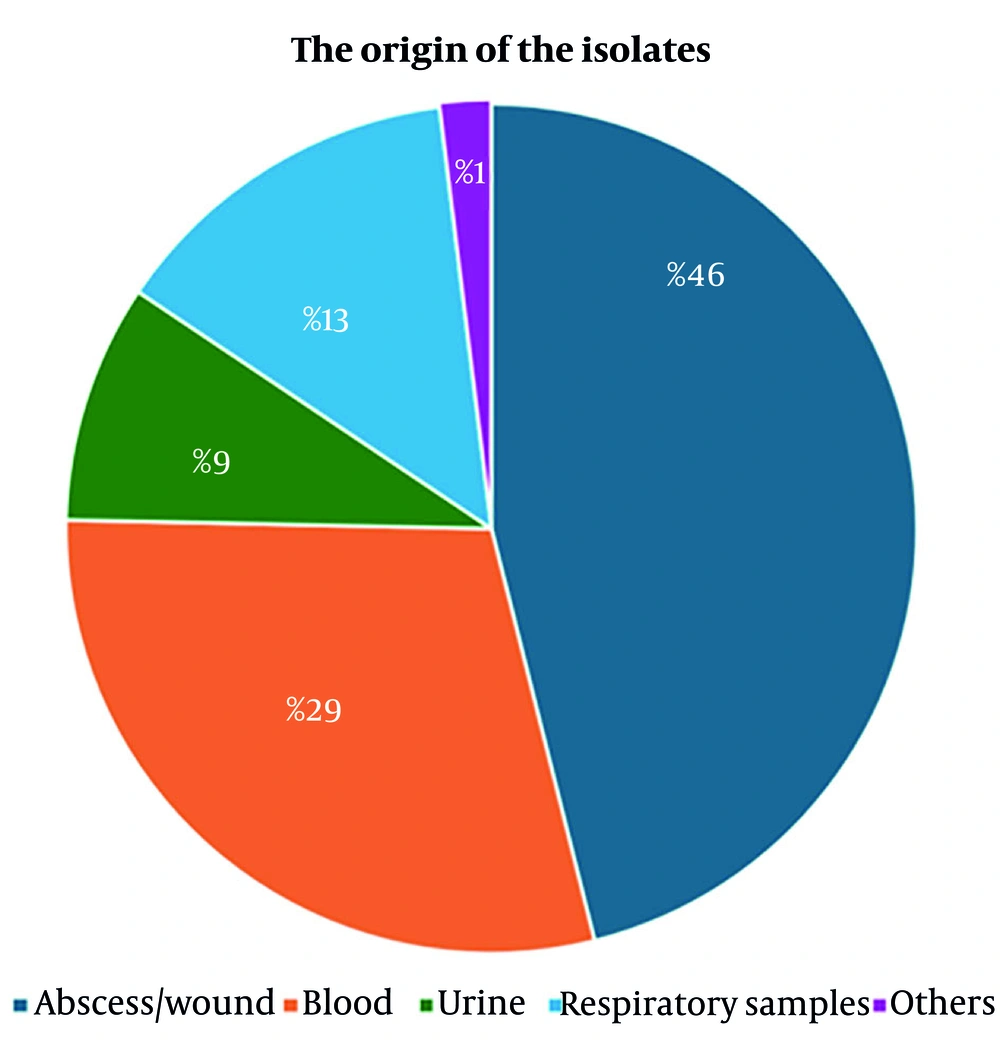
Achromobacter spp. was identified in 212 isolates during the study period. However, only 154 cultures were included in the analysis, as 24 patients were under the age of 18, 21 isolates demonstrated repeating culture positivity, and 13 cultures were deemed colonizations (Figure 1). The median age of the cohort was 52 years, with 68.5% being male. Among the 107 patients, at least one co-morbidity was present, with immunosuppression being the most prevalent (39.6%). The highest rate of isolation (42.9%) was observed from surgical clinics. The most frequently administered antibiotics were piperacillin-tazobactam (25.3%) and meropenem (16.2%). Polymicrobial infections were documented in 50 patients (32.5%). The 28-day mortality rate was 12% (Table 1). Anti-HIV antibody was tested during the hospitalization period, and none resulted in positive. The origin of the isolates is illustrated in Figure 2, showing that 46% originated from abscess and wound samples, 29% from blood, 13% from respiratory specimens, and 9% from urine samples. One percent of the isolates were obtained from samples such as catheter tips or cerebrospinal fluid.
| Variables | Values (N = 154) |
|---|---|
| Age | 52 (18 - 88) |
| Male gender | 105 (68.5) |
| Years | |
| March 2014 to March 2015 | 21 (13.6) |
| March 2015 to March 2016 | 13 (8.4) |
| March 2016 to March 2017 | 14 (10) |
| March 2017 to March 2018 | 12 (7.7) |
| March 2018 to March 2019 | 12 (7.7) |
| March 2019 to March 2020 | 9 (5.8) |
| March 2020 to March 2021 | 20 (12.9) |
| March 2021 to March 2022 | 22 (14.2) |
| March 2022 to March 2023 | 18 (11.6) |
| March 2023 to March 2024 | 13 (8.4) |
| At least one chronic disease | 107 (69.5) |
| Immunosuppression | 61 (39.6) |
| Malignancy | 41 (26.6) |
| Diabetes mellitus | 30 (19.5) |
| Hypertension | 19 (12.3) |
| Coronary artery disease | 19 (12.3) |
| Chronic kidney disease | 18 (11.7) |
| Osteomyelitis | 13 (8.4) |
| Trauma | 21 (13.6) |
| Isolated unit | |
| Surgical clinics | 66 (42.9) |
| Internal clinics | 52 (33.8) |
| Intensive care unit | 41 (26.6) |
| Antibiotic treatments | |
| Piperacillin tazobactam | 59 (38.3) |
| Meropenem | 38 (24.6) |
| Cefepime/Ceftazidime | 16 (10.2) |
| Others | 41 (26.6) |
| Polymicrobial infection | 50 (32.5) |
| 28-day mortality | 18 (11.7) |
General Characteristics of Patients Included in the Study a
Table 2 shows a comparative analysis of the characteristics, laboratory findings, and prognoses of patients with polymicrobial (n = 50) versus monomicrobial (n = 104) infections. The prevalence of having at least one chronic condition was significantly higher among patients with monomicrobial infections (75% vs. 58%; P = 0.032). Additionally, the history of trauma was more prevalent in monomicrobial cases (16% vs. 8%; P = 0.035). The abscess/wound isolate rate in polymicrobial infections was higher than in monomicrobial infections (36% vs. 68%, P < 0.001), whereas monomicrobial infections were more frequently associated with isolates from blood (P = 0.004) and urine (P = 0.034) samples. No significant difference was observed between the groups in the comparison of white blood cells and C-reactive protein levels before and after treatment. The 28-day mortality rate was 17.6% in patients with monomicrobial infections, compared to 4% in those with polymicrobial infections (P = 0.037).
| Variables | Monomicrobial (N = 104) | Polymicrobial (N = 50) | P |
|---|---|---|---|
| Age | 54 (18 - 84) | 45 (18 - 88) | 0.771 |
| Male gender | 70 (67.3) | 35 (70) | 0.973 |
| At least one chronic disease | 78 (75) | 29 (58) | 0.032 |
| Immunosuppression | 46 (44.2) | 15 (30) | 0.091 |
| Malignancy | 31 (29.8) | 10 (20) | 0.197 |
| Diabetes mellitus | 24 (23.1) | 6 (12) | 0.104 |
| Coronary artery disease | 15 (14.4) | 4 (8) | 0.256 |
| Chronic kidney disease | 12 (11.5) | 6 (12) | 0.933 |
| Osteomyelitis | 9 (8.7) | 4 (8) | 0.919 |
| Trauma | 17 (16.3) | 4 (8) | 0.035 |
| Type of isolated sample | |||
| Abscess/wound | 37 (35.6) | 34 (68) | <0.001 |
| Blood | 38 (37) | 7 (14) | 0.004 |
| Urine | 13 (12.5) | 1 (2) | 0.034 |
| Respiratory samples | 12 (11.5) | 9 (18) | 0.274 |
| Type of units | |||
| Medical units | 35 (34) | 17 (34) | 0.966 |
| Surgical units | 44 (42) | 22 (44) | 0.842 |
| Intensive care units | 30 (28) | 11 (22) | 0.368 |
| Pre-treatment laboratory | |||
| White blood cell | 8855 (390-22560) | 10265(4720-17600) | 0.446 |
| C-reactive protein | 72 (1-370) | 67 (1-235) | 0.775 |
| Post-treatment laboratory | |||
| White blood cell | 7110 (110-34000) | 8365 (3520-17970) | 0.443 |
| C-reactive protein | 30 (1-295) | 29 (1-88) | 0.465 |
| 28-day mortality | 16 (17.6) | 2 (4) | 0.037 |
Comparison of the Characteristics and Prognoses of Patients with Polymicrobial and Monomicrobial Infections a
When comparing bacteremic (n = 45) and non-bacteremic (n = 109) patients, the incidence of hypertension (22% vs. 8.3%; P = 0.017) and coronary artery disease (45% vs. 7.3%; P = 0.003) was significantly higher in those with bacteremia. The prevalence of bacteremia was significantly higher in samples obtained from intensive care units (48% vs. 17%, P < 0.001). Conversely, the rate of polymicrobial infection was more pronounced in non-bacteremic patients (P = 0.004). In terms of antibiotic regimens, the utilization of meropenem was significantly higher in bacteremic patients (44% vs. 16%; P = 0.006). Although the 28-day mortality rate was higher among bacteremic patients (20% vs. 10.5%), this difference did not reach statistical significance (Table 3).
| Variables | Bacteremic (N = 45) | Non-bacteremic (N = 109) | P |
|---|---|---|---|
| Age | 55 (18 - 84) | 50 (18 - 88) | 0.856 |
| Male gender | 25 (55.6) | 80 (73.4) | 0.031 |
| At least one chronic disease | 33 (73) | 74 (68) | 0.505 |
| Immunosuppression | 18 (40) | 43 (39.4) | 0.949 |
| Malignancy | 13 (29) | 28 (25.7) | 0.683 |
| Diabetes mellitus | 13 (29) | 17 (15.6) | 0.058 |
| Hypertension | 10 (22) | 9 (8.3) | 0.017 |
| Coronary artery disease | 11 (45) | 8 (7.3) | 0.003 |
| Chronic kidney disease | 8 (17.8) | 10 (9.2) | 0.131 |
| Osteomyelitis | 1 (2.2) | 12 (11.4) | 0.015 |
| Trauma | 4 (8.9) | 17 (15.6) | 0.270 |
| Type of units | |||
| Medical units | 19 (42.2) | 33 (30.3) | 0.154 |
| Surgical units | 5 (11) | 61 (56) | < 0.001 |
| Intensive care units | 22 (48.9) | 19 (17.4) | < 0.001 |
| Polymicrobial infection | 7 (15.6) | 43 (39.4) | 0.004 |
| Antibiotic treatments | |||
| Piperacillin tazobactam | 20 (44.4) | 39 (35.7) | 0.569 |
| Meropenem | 20 (44.4) | 18 (16.6) | 0.006 |
| Cefepime/ceftazidime | 6 (13.3) | 10 (9.2) | 0.955 |
| Others | 14 (31.1) | 27(24.7) | 0.325 |
| 28-day mortality | 8 (20.0) | 10 (10.5) | 0.139 |
Comparison of Clinical Features, Treatments and Prognosis of Bacteremic and Non-bacteremic Patients a
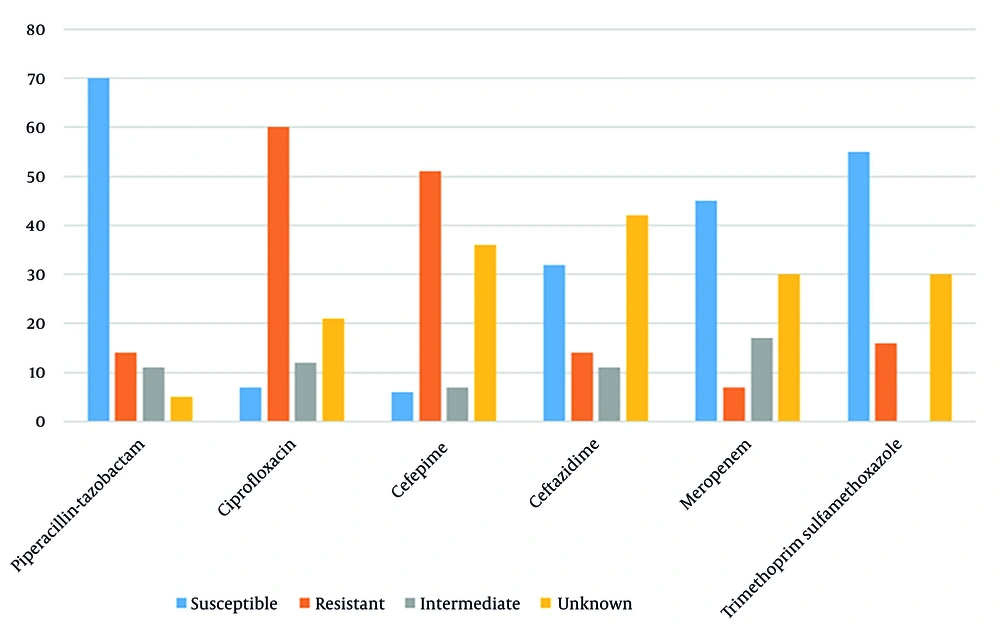
The antimicrobial susceptibilities of isolates are illustrated in Figure 3. Piperacillin-tazobactam exhibited the highest susceptibility rate (70%), followed by imipenem (60%). Resistance to cephalosporins exceeded 50%.
5. Discussion
In this study, 69.5% of patients presented with at least one chronic condition. The rate of immunosuppression was 40%, while malignancy was observed in 26% of the cohort. A laboratory-based surveillance study conducted by Işler et al. in Australia reported that 90% of patients with 195 bloodstream infections had a Charlson Comorbidity Index (CCI) score of 1 or higher, with 19% having a CCI score of 3 or higher (5). The group most frequently affected by Achromobacter spp. infections comprises patients with cystic fibrosis (17, 18), in whom bacterial colonization is likely due to persistent inflammation in the respiratory tract. Furthermore, Achromobacter strains can persist in the respiratory tract through various patho-adaptive mutations, such as biofilm formation, genetic diversification, immune evasion, and the development of antibiotic resistance (19).
In the literature, Achromobacter spp. has been identified as the causative agent of bacteremia in patients with chronic myeloid leukemia (20), cellulitis following allogeneic hematopoietic stem cell transplantation (12), and bacteremia following endoscopic retrograde cholangiopancreatography in individuals with cholangiocarcinoma (21). Achromobacter spp. should be considered a possible pathogen in immunocompromised patients due to its opportunistic character. The incidence of polymicrobial infections was more pronounced in samples procured from abscesses. A meta-analysis that investigated surgical site infections attributable to Achromobacter spp. revealed concomitant infections with bacteria such as Escherichia coli, Morganella morganii, Acinetobacter baumannii, and Stenotrophomonas maltophilia, particularly following intra-abdominal, neurological, thoracic, and gynecological procedures (22).
In a retrospective study of Achromobacter spp. infections in cystic fibrosis patients, it was noted that this bacterium ranked as the 5th most prevalent pathogen and frequently contributed to co-infections in the context of chronic Pseudomonas aeruginosa and Burkholderia cepacia complex infections (23). Similarly, S. maltophilia and P. aeruginosa were frequently identified as co-infecting organisms in a retrospective analysis of ventilator-associated pneumonia (VAP) caused by Achromobacter spp., where 84% of patients exhibited polymicrobial VAP (24). It is imperative to recognize that Achromobacter spp. can also behave as an opportunistic pathogen, playing a significant role in polymicrobial infections, especially in surgical site and abscess specimens.
In this study, the observed 28-day mortality rate was 11.7%, with mortality being higher in patients with monomicrobial infections. Given that these patients predominantly presented with bloodstream infections, it was hypothesized that the incidence of septic manifestations may have been elevated in this group. A retrospective analysis of 14 patients with Achromobacter spp. infections reported that those presenting with septic symptoms were bacteremic, and that the three patients who succumbed were also bacteremic and had presented with sepsis (4). Additionally, it was documented that mortality in patients with Achromobacter spp. infections following lung transplantation (27%) exceeded that of patients without such infections (12%) (6). When Achromobacter spp. was implicated as the etiologic agent in VAP, the mortality rate was reported to be 9% (24).
In a meta-analysis by Ronin et al. that evaluated surgical site infections caused by Achromobacter spp., it was reported that cases with mortality were mostly seen after complications and complicated surgery that led to mediastinitis, peritonitis, or endocarditis (22). Mortality rates were observed to fluctuate based on the clinical presentation and the site of infection; however, they remained elevated across all cases. Prompt identification of the infectious pathogen and its antimicrobial resistance, followed by the application of appropriate treatment, is crucial.
In this study, when comparing 45 bacteremic patients to those without bacteremia, it was observed that chronic conditions such as hypertension and coronary artery disease were more prevalent among bacteremic individuals, and the majority of these patients were managed in intensive care units. In instances of bacteremia outbreaks linked to contaminated disinfectants or pharmaceuticals, affected patients predominantly had chronic conditions, such as hematological malignancies or required hemodialysis (25, 26). In an observational study by Siddiqui et al. (4), it was reported that all bacteremic patients had either a hematological malignancy or an autoimmune disease. Although the difference in mortality was not statistically significant, a higher mortality rate was noted among bacteremic patients. Meropenem treatment rates were higher in patients with bacteremia, but mortality was still higher in this patient group. Considering the resistance rates, the fact that the mortality rate was higher despite the use of appropriate antibiotics indicates that other parameters such as patients' ICU severity scores and source control should also be taken into consideration.
In the present study, resistance rates to cefepime and ceftazidime were observed to exceed 50% in Achromobacter spp. isolates, whereas the lowest resistance rates were noted for piperacillin, tazobactam, and carbapenems. In the report by Marion-Sanchez et al., 59% of isolates from hospital-acquired infections exhibited fluoroquinolone resistance, while 38% were categorized as intermediate (2). In a 2014 analysis of antimicrobial susceptibility from isolates of 109 cystic fibrosis patients in France, the majority were found to have acquired fluoroquinolone resistance, with additional acquired resistance mechanisms reported for piperacillin, tazobactam, carbapenems, and ceftazidime-avibactam, particularly in A. xylosoxidans isolates (27). Carbapenem resistance was reported to exceed 50% in bacteremic patients (28).
When devising an empirical treatment regimen for infections caused by Achromobacter spp., factors such as the infection site, the severity of the disease, and regional resistance patterns must be carefully considered. Based on the resistance data from our region, particular caution should be exercised when empirically administering aminoglycosides, cephalosporins, and fluoroquinolones.
5.1. Conclusions
This extensive study represents a thorough examination of Achromobacter spp. isolates procured from a tertiary healthcare facility. It is imperative to acknowledge that polymicrobial agents may frequently underline surgical site infections, while bacteremia, particularly prevalent in intensive care units, poses a significant risk. Understanding local antimicrobial resistance profiles is paramount for the initiation of targeted and effective empirical therapies. Given the resistance patterns observed, piperacillin-tazobactam or carbapenems should be considered first-line options when selecting empirical treatment strategies, thereby optimizing therapeutic outcomes and mitigating resistance-related complications.
5.2. Limitations
The main limitation of this study is that it was designed in a single center and retrospectively. Since identification methods and antibiotics used for sensitivity can change over the years, there may be gaps in the data over a 10-year period. In addition, the inability to classify Achromobacter at the genus level is another limitation of this study. The transition from CLSI to EUCAST during the study period may create uncertainty in resistance rates. However, in the stored strains that could be revived, susceptibility was studied according to EUCAST standards and current data were used.