1. Background
Leptin, an adipose-derived hormone/cytokine, is a powerful defending element of Immune system against bacterial infections (1, 2). Congenital leptin deficiency causes immune dysfunction in affected newborns and is associated with early childhood death due to overwhelming infections (2, 3). Administration of leptin to leptin deficient mice has been shown to make them resistant to Klebsiella pneumonia (4). Leptin stimulates the proliferation of peripheral monocytes in-vitro and leads to enhanced expression of CD11b, a marker of neutrophilic phagocytosis, on the surface of monocytes (5, 6). Despite the potent role of leptin in immune system functions, its interaction with diverse pathogenic bacteria, especially intracellular bacteria which potentially escape from phagocytosis by immune cells, is not well understood.
Listeria monocytogenes is an intracellular pathogen which causes various diseases such as fulminant Listeriosis, meningitis, endocarditis and disseminated bacteremia mainly in immune-deficient patients and neonates (2, 7,8). Listeriolysin secreted by L. monocytogenes, inhibits the procedure of macrophage-mediated processing of bacterial antigens (9, 10). Indeed, L. monocytogenes destructs cell membranes especially phagosomemembranes thatleads to intracellular bacterial growth (9, 10). L. monocytogenes potently induces apoptosis in T-cell lymphocytes through both caspase-dependent and independent pathways (11, 12). After phagocytosis of L. monocytogenes by antigen-presenting cells (APC), the produced Listeriolysin O potentiates its cytosolic growth which leads to APC death through inflammatory mediators (13, 14). Due to the diverse effects of this pathogen on induction of apoptosis and phagocytosis of immune cells and its ability to escape from immune system, L. monocytogenes was selected to be used in this study (8).
2. Objectives
The aim of this study was to investigate the role of leptin in induction of phagocytosis and apoptosis on cells of immune systems infected by L. monocytogenes. Since Escherichia coli has been shown to enhance the CD11b expression on the surface of neutrophils triggered by the presence of FMLP, E. coli was used as a control bacteria in this study (15).
3. Materials and Methods
10-15 mL of heparinized blood were drawn from healthy male volunteers (age 21-34) after they have signed informed contest form. The study was approved by local ethics committee. To evaluate the blood neutrophils, 100 µL of blood was transferred into each sterile flow cytometry tube. Samples were treated differently as listed in Table 1. L. monocytogenes PTCC1295 and non-pathogenic E. coli were grown in TSA/TSB (Merck, Germany) culture medium supplemented with 1% yeast extract (Merck, Germany) and 10% human blood and Nutrient agar (Merck, Germany )culture medium, respectively. L. monocytogenes
was used for stimulation of PBMCs (Peripheral Blood Mononuclear cells) compared with E. coli as a control. For bacterial challenge, 108 bacteria were added to 10 6 cell (multiplicity of infection=100).
Leptin (R&D systems, USA) was used at a concentration of 250ng/mL. After treatments, tubes were incubated in thermomixer for 10 min at 37°C and incubation was carried out for 3 hours. Cells were incubated with 5 µL of listed antibodies for 20 min at 40°C in dark. Then, RBCs were lysed using RBC lysis buffer (DAKO 53350, Germany). The neutrophilsCD11b expression, as anactivity and phagocytosismarker, was evaluated using flow cytometry (Partec PAS, Germany) after staining with FITC-conjugated murine anti-human CD11b monoclonal antibody (Serotec, England). FITC-conjugated murine IgG1 monoclonal antibodies (Serotec, England) were used as staining control antibody. Data were analyzed using Flow Max software (Partec, Germany). All experiments were repeated 15 timesFor assessment of apoptosis, 10 mL of blood was used for isolation of mononuclear cells (PBMCs) from peripheral blood using Histopaque (Sigma A6929, Germany) centrifuged at 400g for 30 min according to manufacturer’s recommendations.
After gentle mixing of isolated peripheral blood mononuclear cells with phosphate buffer saline (PBS, CMG Iran), cells were centrifugated at 250g for 10 min. Then, cells were transferred into RPMI-1640 culture media (Sigma R8755, Germany) and were washed twice. Isolated PBMCs were counted using improved Neobarhaemocytometer. To confirm and assess the presence of T-lymphocyte, cells were labeled with anti-human CD3-antibody (BD Pharmingen, USA) and were counted by flow cytometry.
| Treatments Agent(s) | Staining Antibody |
|---|---|
| PBS | Without antibody |
| PBS | FITC-conjugated murine IgG1 monoclonal antibody |
| PBS | FITC-conjugated murine anti-human CD11b monoclonal antibody |
| E. Coli | Without antibody |
| E. Coli | FITC-conjugated murine IgG1 monoclonal antibody |
| E. Coli | FITC-conjugated murine anti-human CD11b monoclonal antibody |
| L. monocyogenes | Without antibody |
| L. monocyogenes | FITC-conjugated murine IgG1 monoclonal antibody |
| L. monocyogenes | FITC-conjugated murine anti-human CD11b monoclonal antibody |
| Leptin+ E. coli | Without antibody |
| Leptin+ E. coli | FITC-conjugated murine IgG1 monoclonal antibody |
| Leptin+ E. coli | FITC-conjugated murine anti-human CD11b monoclonal antibody |
| Leptin+ L. monocyogenes | Without antibody |
| Leptin+ L. monocyogenes | FITC-conjugated murine IgG1 monoclonal antibody |
| Leptin+ L. monocyogenes | FITC-conjugated murine anti-human CD11b monoclonal antibody |
After treatment of cells according to Table 2, cells were incubated in thermomixer for 10 min at 37°C. Allowing the inoculated bacteria to exert their effects on cells, cells were incubated for 24 h at 37°C. Doxorubicin, Germany (50 mg/25 mL) was used as a positive control for induction of cell apoptosis. All experiments were repeated 12 times. For analysis of apoptosis, cells were incubated with FITC-conjugated Annexin V (BD Pharmingen, USA) and PI (BD Pharmingen, USA) for 30 min at 40°C and were analyzed by flow cytometry (Partec PAS, Germany), data were analyzed using Flow Max software. For statistical analysis, t-test via SPSS v.17 was used. The list of treatments for apoptosis is demonstrated in Table 2.
| Treatments | Staining agent |
|---|---|
| No treatment | FITC-conjugated Annexin V |
| No treatment | PI |
| Doxorubicin | FITC-conjugated Annexin V |
| Doxorubicin | PI |
| E. Coli | FITC-conjugated Annexin V |
| E. Coli | PI |
| L. monocyogenes | FITC-conjugated Annexin V |
| L. monocyogenes | PI |
| Leptin+ E. coli | FITC-conjugated Annexin V |
| Leptin+ E. coli | PI |
| Leptin+L. monocyogenes | FITC-conjugated Annexin V |
| Leptin+L. monocyogenes | PI |
4. Results
4.1. Effects of Leptinon CD11b Expression
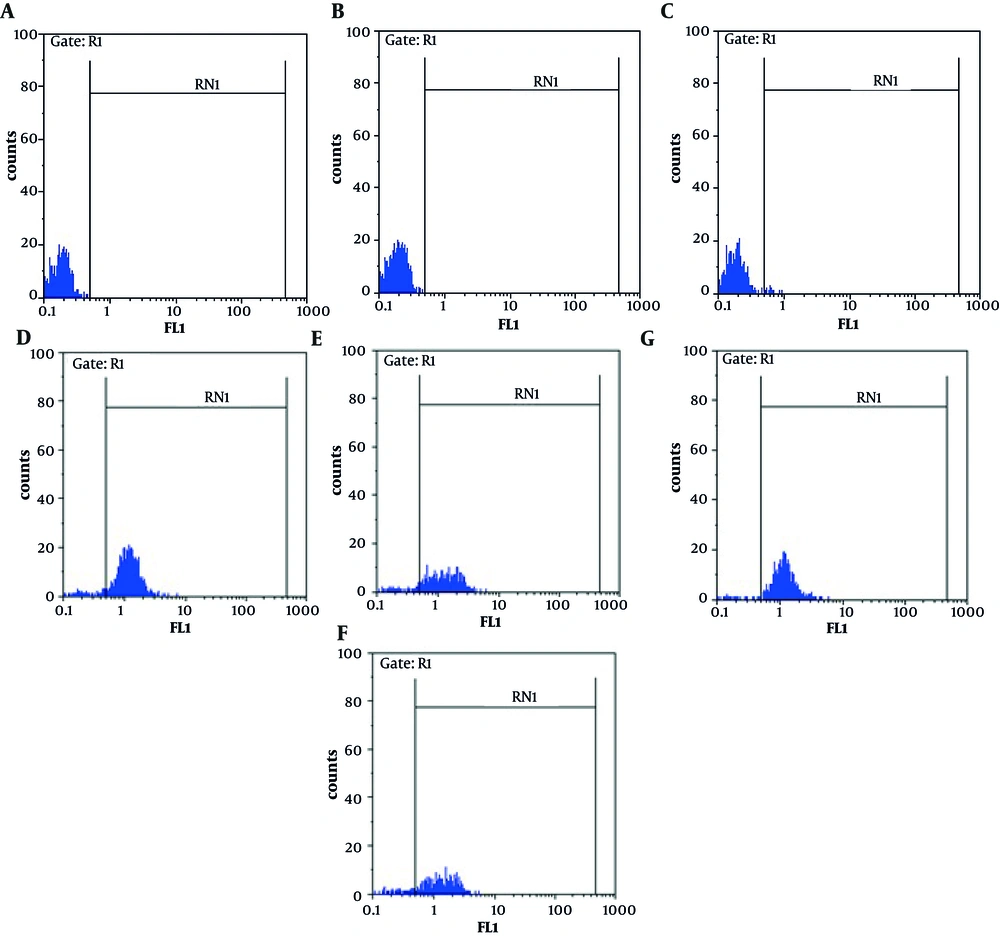
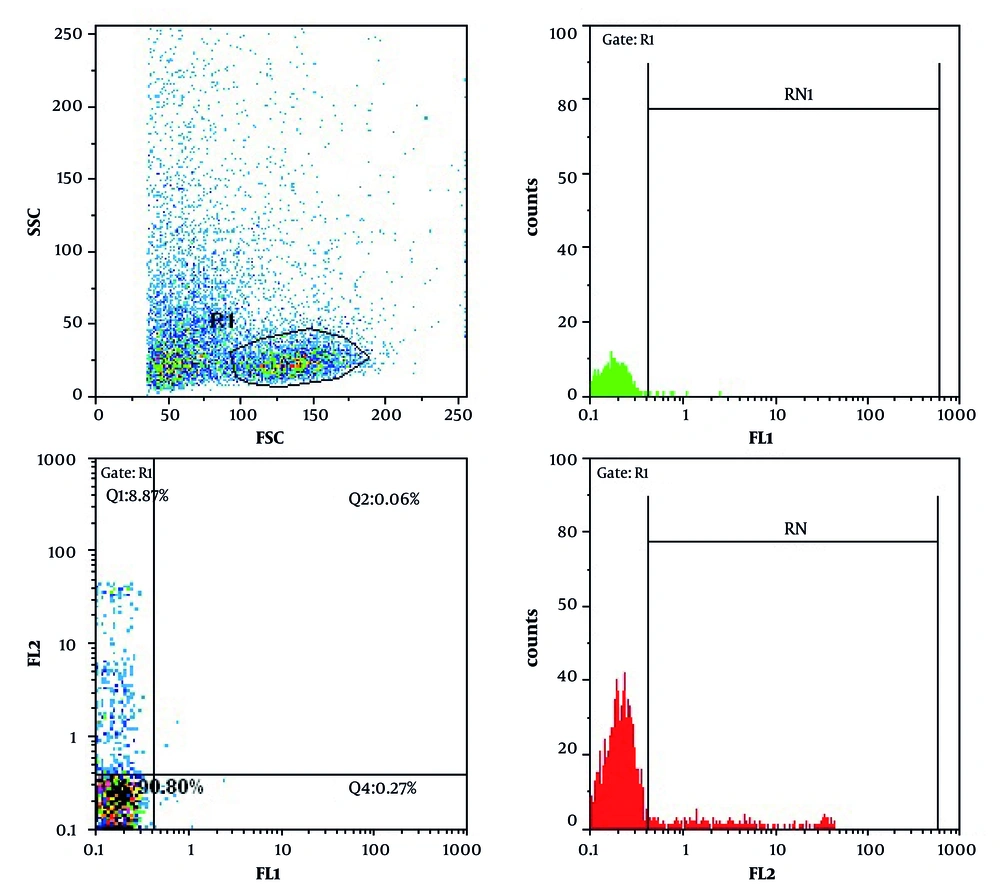
CD11b expression on the surface of peripheral blood neutrophils indicates the occurrence of phagocytosis leading to neutrophil stimulation. Data in Figure 1 show the expression of CD11b after various treatments.After 24 h, 0.27%, 8.87% and 90.80% of non-treatedlymphocytes were apoptotic, necrotic and viable, respectively (Figure 2).
4.2. Effects of Leptin on Induction of Apoptosis
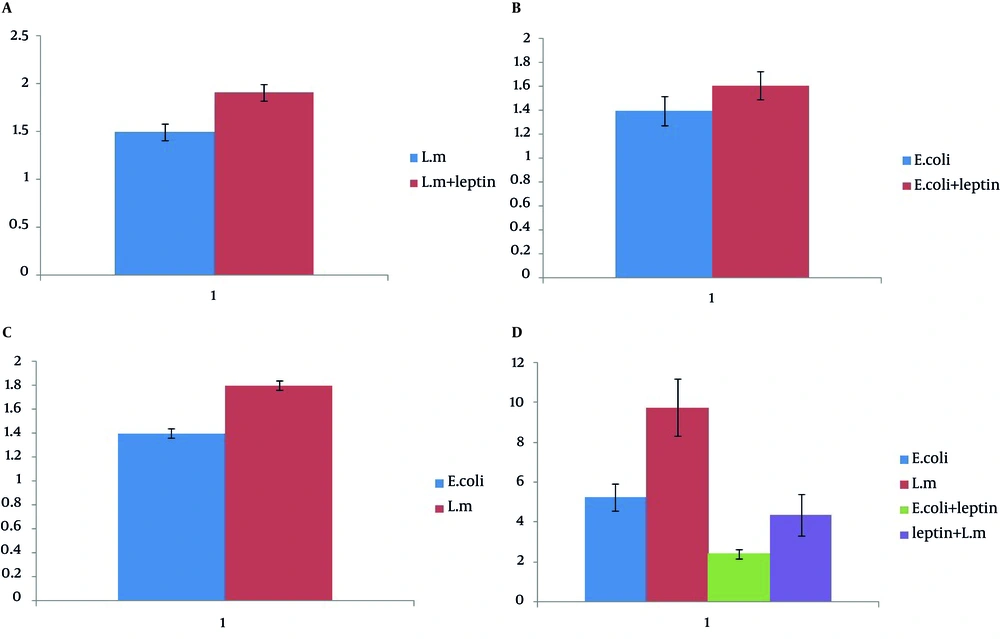
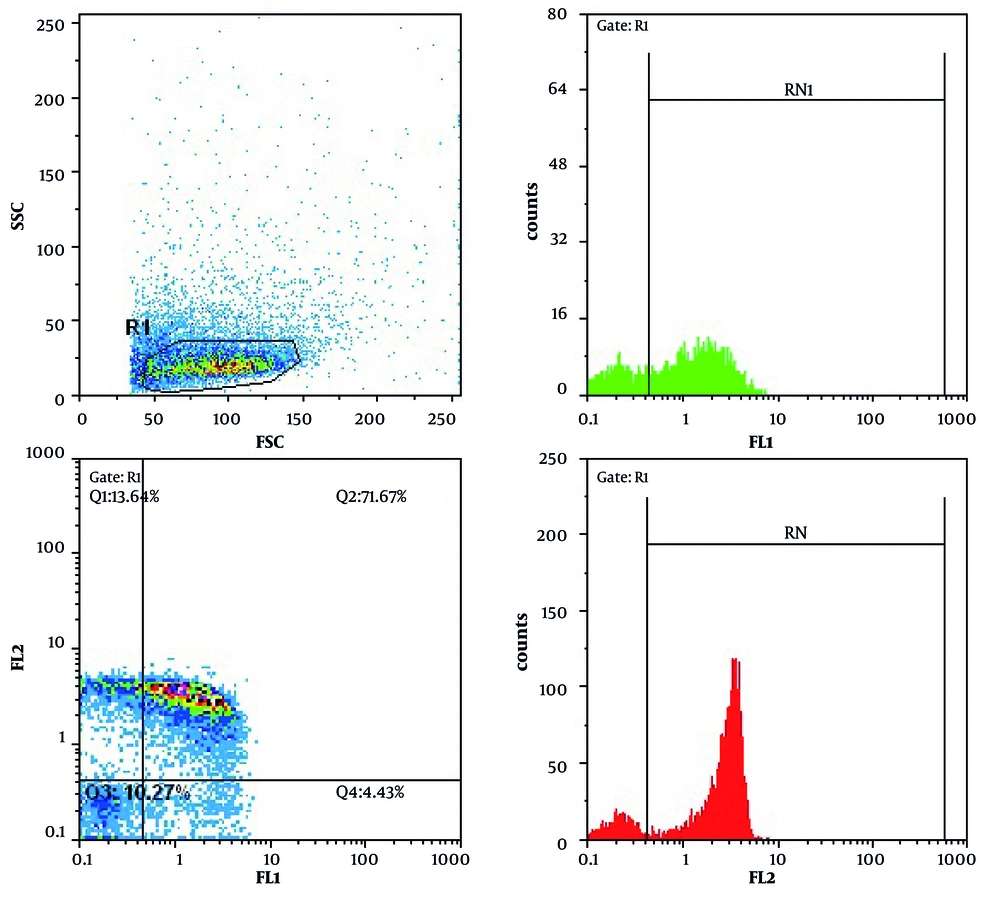
In Doxorubicin treated cells (as a control), after 24 h, 43.04% and 53.60 of cells were apoptotic and viable, respectively. Doxorubicin potently induced apoptosis in treated cells. The percentage of apoptotic cells treated with leptin, E. coli and L. monocytogenes were 2.62%, 4.43% and 7.94%, respectively, after 24 h. L. monocytogenes induced apoptosis more potent than leptin and E. coli(P<0.05). Cell treated with leptin and L. monocytogenes after 24 h showed less apoptosis rate (4%) than only L. monocytogenes treated cells (P < 0.05).
Table 3 demonstrates the rate of apoptosis induction on lymphocytes after various treatments. Data in Figure 3 indicates the rate of apoptosis in neutrophils treated with L. monocytogenes , E.coli , leptin+ L. monocytogenes and leptin+ E. coli and E. coli , E. coli + Leptin, L. monocytogenes and L. monocytogenes + leptin. Figures 3 and 4 illustrate the percentage of apoptosis, necrosis, apoptosis and necrosis and viability of cells treated with L. monocytogenes , E. coli , leptin + L. monocytogenes , leptin + E. coli and E. coli , leptin+ L. monocytogenes and L. monocytogenes.
| Treatment | Apoptosis, Four Replicates |
|---|---|
| E. coli | 5.2675% |
| L. monocytogenes | 9.7825% |
| Leptin plus E. coli | 2.4275% |
| Leptin plus L. monocytogenes | 4.3775% |
This graph demonstrates CD11b expression on the surface of neutrophils treated with a- L. monocytogenes and E. coli (as a control), b- Leptin+ E. coli and E. coli, c- Leptin+ L. monocytogenes and L. monocytogenes, d- E. coli, E. coli + Leptin, L. monocytogenesand L. monocytogenes + Leptin. Data are expressed as Mean ± SD. * indicates statistically significant difference in phagocytosis between two groups (P<0.05). The X axis shows two different bacteria and the Y axis demonstrate the Mean Fluorescent Intensity (MIF) of Cd11b expression. Data are derived from six replicates.
5. Discussion
The major finding of this investigation is that L. monocytogenes enhances the neutrophilic phagocytosis more than E. coli. L. monocytogenes is an intracellular pathogen which is able to escape from immune systemand phagosomes through secretion of Listeriolysin. The principal cause of enhanced CD11b expression is the neutrophil stimulation by this toxin. The mechanism of apoptosis by Listeriolysin has been demonstrated to be mediated by CR3-receptor (CD11b and CD18) which play the principal role in bacterial eradication (15, 16). The potent stimulation of phagocytosis by L. monocytogenes compared with E. coli, based on our data, is due to its ability to stimulate CD11b expression more effectively than E. coli. Regarding bacterial phagocytosis, our data demonstrates enhanced neutrophilic phagocytosis and CD11b expression on the surface of neutrophils stimulated by L. monocytogenes and E. coli.
Leptin involves in modulation of neutrophilic phagocytosis after binding to Ob-R receptor expressed on the surface of neutrophils (5). Leptin stimulates neutrophils and enhances accumulation of reactive oxygen within neutrophilicphagosomes (5, 17). In this way, leptin enhancesneutrophilic phagocytosis (18, 19). Leptin actively participates in formation of phagosomes through stimulation of actin polymerization and modulation of actinomyosine interaction (20). Knock-out mice for leptin hormone, showed defective phagocytosis responses to Klebsiella pneumonia. Neutrophils in complement-rich and leptin-deficient environment showed a decreased expression of CD11b which became normalized by leptin treatment (4).
Leptin role in induction of phagocytosis is CD11b-dependent. Leptin deficiency is associated with enhanced susceptibility to bacterial infection (21). Zarkesh-Esfahani et al. have demonstrated that leptin, by itself, was not able to stimulate CD11b expression on the surface of neutrophils,while addition of monocytes to neutrophils enhanced CD11b expression in the presence of leptin through stimulation of neutrophils by secreted TNF-α (6). Then, leptin can be used to enhance neutrophilic phagocytosis in bacterial infections such as L. monocytogenes. Leptin enhanced phagocytosis of L. monocytogenes more potent than E. coli.
Induction of apoptosis in murine lymphocytes by L. monocytogenes has been demonstrated by Carro et al. in which Listeriolysin O, as a virulent factor, has been considered todirectly induce apoptosis at very low concentrations (22). Injection of L. monocytogenes into mouth, caused wound creation in lymphatic tissue and aggregation of macrophages and neutrophils accompanied by dramatic decrease in the number of T lymphocytes (23). The data demonstrate that L. monocytogenes induced apoptosis more potent than E. coli, which is compatible with previous reports. This might be due to the presence of apoptotic factor Listeriolysin O, which is absent in E. coli. In control lymphocytes only treated with leptin, attenuated lymphocytic apoptosis was observed. Leptin has been shown to decrease lymphocyte apoptosis rate through inhibition of FAS-directed apoptosis pathway (24).
B-cell lymphocytes are more susceptible to induce the apoptosis by leptin related to greater expression of leptin receptor on their surface compared with T-cell lymphocytes (24). Continuous leptin injection into the starved mice has been associated with decreased lymphocyte loss through inhibition of cell apoptosis (25). Leptin injection into mice with enhanced lymphocyte apoptosis as a consequence to steroid administration, has reversed the process (25). Induced lymphocytic apoptosis by leptin is implicated in potentiating immune defense against virulent pathogens. Our data regarding the effects of leptin on induction of apoptosis is compatible with previous findings. Interestingly, our data demonstrates accentuated apoptosis induction after treatment of L. monocytogenes activated lymphocytes by leptin.
Leptin can be used as a potent agent for an effective induction of bacterial phagocytosis and lymphocytic apoptosis in the cases with sever immune-deficiency. It can also be used for the treatment of severe and intractable L. monocytogenes and E. coli infections.