1. Background
Streptomyces avermitilis belongs to Streptomycetes which is a group of Gram positive bacteria having circular genome and form aerial spores (1). It produces macrocyclic lactones, the oligomycin (2) and secondary metabolites, the Avermectin. The avermectin produced by this bacterium could be used as anthelmintic and insecticidal agent (3, 4). It is 16-membered pentacyclic compounds having polysaccharide of methylated deoxysugar L-oleandros polyketide (5).
Ivermectin, selamectin, doramectin and abamectin are the compounds that have been derived from avermectin and are used as anthelmintic agents. They show great activity against nematodes and arthropod parasites of domestic animals when given a dose with rate of 300 µg/kg or less. However they do not show activity against bacteria and fungi as do the other macrolides and polyene antibiotic (6). The main function of avermectins is that they block the transmittance of electrical activity in nerves and muscle cells by stimulating the release and binding of gamma-aminobutyric acid (GABA) at nerve endings. As a result of this the chloride ions enter into the cells resulting in hyperpolarization and paralysis of the neuromuscular systems (7).
Different species of Streptomyces produce avermectin by fermentation process using different medium. Nutritional requirements are very crucial during the secondary metabolite production (8). Carbon (C) and nitrogen (N) are the main components required for the production of avermectin from S.avermitilis by fermentation (9, 10) When a mixture of different carbon sources is provided, the microorganism will utilize only C-source that is easily metabolized. In many cases glucose is supposed to be a represent for secondary metabolite production (11).
Starch is the best C-source used for the production of avermectin (12-14). Similarly nitrogen source also plays an important role in avermectin production. During fermentation process nitrogenous components release very slowly (15). Yeast extract is the best nitrogen source utilized by Streptomyces for the production of antibiotics (16, 17). It is associated with greatest mycelium production and growth (18). However increase in N-source level will have inhibitory effect on avermectin B1a production (19).
2. Objectives
The present study was conducted to select an appropriate medium for the maximum yield of avermectin B1b from S. avermitilis 41445 in laboratory scale shake flask submerged fermentation.
3. Materials and Methods
3.1. Microorganism and Maintenance
The strain S. avermitilis 41445 was used for the production of avermectin B1b throughout the study. The strain S.avermitilis 41445 was maintained on medium 65 as specified by DSM. The medium 65 (YMG medium) consisted of (g/L in distilled water) glucose 4.0, yeast extract 4.0, malt extract 10.0 and CaCO3 2.0. The pH of the medium was adjusted to 6.5 before autoclave. The pH was adjusted at 7.0 with CaCO3 after autoclaving the medium at 121°C for 15 min. The medium was then inoculated with S. avermitilis 41445 and incubated at 28°C in water bath shaker at 150 x g until the brownish liquid was obtained. Culture was then streaked on nutrient agar slants and incubated at 28°C for 24 h for further studies.
3.2. Inoculum
One loopful of 24 h old culture of S. avermitilis 41445 was transferred into the sterilized inoculum medium. Thereafter the inoculum medium was placed at 31°C in the water bath shaker (Eyela, Japan) for 24 h at 150 x g. The seed medium consisted of (g/L in distilled water) glucose 4.0 (Merk Germany), yeast extract 4.0 (Sigma Aldrich chemie, Germany), malt extract 10.0 (Sigma Aldrich chemie, Germany) and CaCO3 2.0 (Merk Germany).
3.3. Production of Avermectin B1b
The production of avermectin B1b by S. avermitilis 41445 was studied into eight different growth media. Each growth medium was inoculated with 2 ml (4% v/v) of inoculum medium separately. After transforming of seed medium, each growth medium was incubated at 31°C in water bath shaker for 15 days at x g. The composition of each fermentation media is given in Table 1.
| Components, g/L | SM1(Self constructed medium) | SM2(Self constructed medium) | SM3(Self constructed medium) | SM4(Self constructed medium) | M1 (Hong Gao, et al. 2009) | M2 (Rezanka, et al. 2007) | M3(Wang, et al. 2010) | M4(DSMZ specified) | |
|---|---|---|---|---|---|---|---|---|---|
| Glucose | 10.0 | - | 10.0 | - | - | 3.0 | - | 4.0 | |
| Soluble starch | - | 50.0 | - | 50.0 | 140.0 | - | 70.0 | - | |
| α-amylase | - | 0.1 | - | 0.1 | - | - | - | - | |
| K2HPO4 | 0.5 | - | 0.5 | - | - | 0.5 | 0.5 | - | |
| CaCO3 | - | 0.8 | - | 0.8 | 0.8 | 5.0 | 2.0 | 2.0 | 2.0 |
| KCl | 0.1 | 0.1 | 0.1 | 0.1 | - | - | 4.0 | - | |
| Yeast extract | - | 2.0 | - | - | 10.0 | 4.0 | 16.0 | 4.0 | 4.0 |
| MgSO4.7H2O | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.1 | 0.5 | - | |
| NaCl | 0.5 | 0.5 | 0.5 | 0.5 | - | 2.0 | - | - | |
| (NH4)2SO4 | - | - | 1.0 | - | 0.25 | 2.0 | - | - | |
| Malt extract | - | - | - | - | - | - | - | 10.0 | 10.0 |
| CoCl2.6H2O | - | - | - | - | - | - | 10.0 | - | |
| Soya flour | - | - | - | - | 28.0 | - | - | - | |
| MnSO4.H2O | - | - | - | - | 0.002 | 0.05 | - | - | |
| FeSO4.7H2O | - | - | - | - | - | 0.1 | - | - | |
| ZnSO4.7H2O | - | - | - | - | - | 0.1 | - | - | - |
apH of each medium was adjusted at 7.2 ± 0.2. All the experiments were performed in the shake flasks containing 50 mL of fermentation medium separately.
3.4. Effect of Process Parameters
Effect of inoculum sizes ranging from 4 to 16% v/v and incubation time ranging from 6 to 15 days were studied on the production of avermectin B1b from S. avermitilis 41445 by fermentation. The effects of temperature and pH were also optimized for the maximum production of avermectin B1b.
3.5. Extraction of Avermectin
Fermentation broth of each flask was centrifuged at 4°C for 20 minutes at 8000 x g. As avermectin is intracellular molecule so cell biomass was taken and supernatant was discarded. The cell biomass in the form of pallet was mixed with appropriate amount of methanol to completely dissolve it. The mixture was centrifuged again and the supernatant was collected for the analysis of avermectin by TLC.
3.6. TLC Analysis of Avermectin
The qualitative analysis of avermectin B1b was performed by applying the supernatant on TLC plate. The plates were then kept in the TLC tank having mobile phase methanol: acetonitrile (Merk, Germany) (98:2 v/v). Short wave ultra violet light (254 nm) was used to detect avermectin on plate as described by (20) after a slight modification in mobile phase.
3.7. HPLC Analysis of Avermectin
The concentration of avermectin B1b components were determined quantitatively by reverse phase HPLC. In HPLC 20 µl of the sample was injected. The samples were separated on C18 column and were eluted by methanol : acetonitrile (98:2 v/v) at a flow rate of 0.5 ml/min with a UV absorbance at 246 nm (21).
4. Results
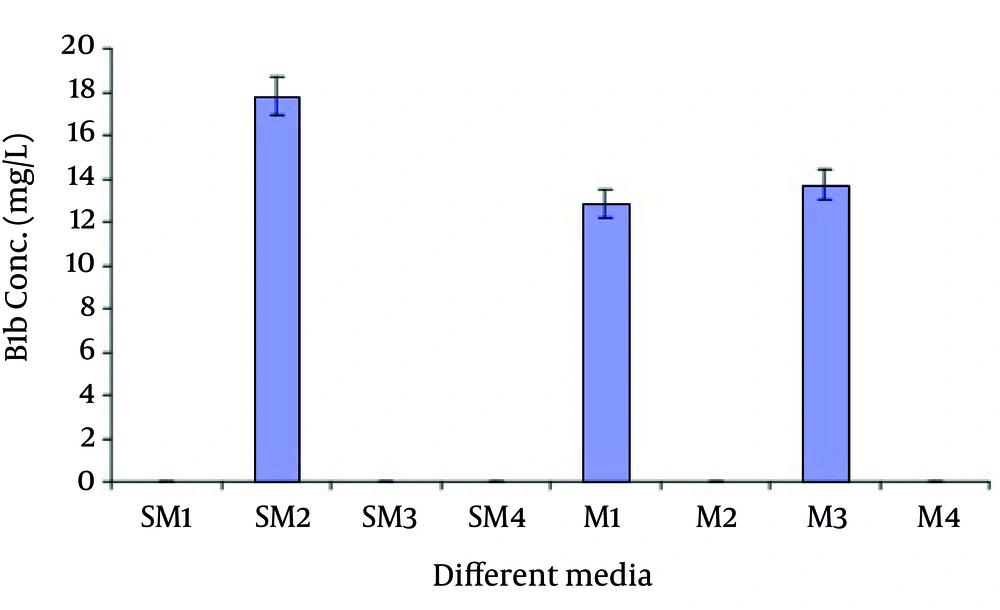
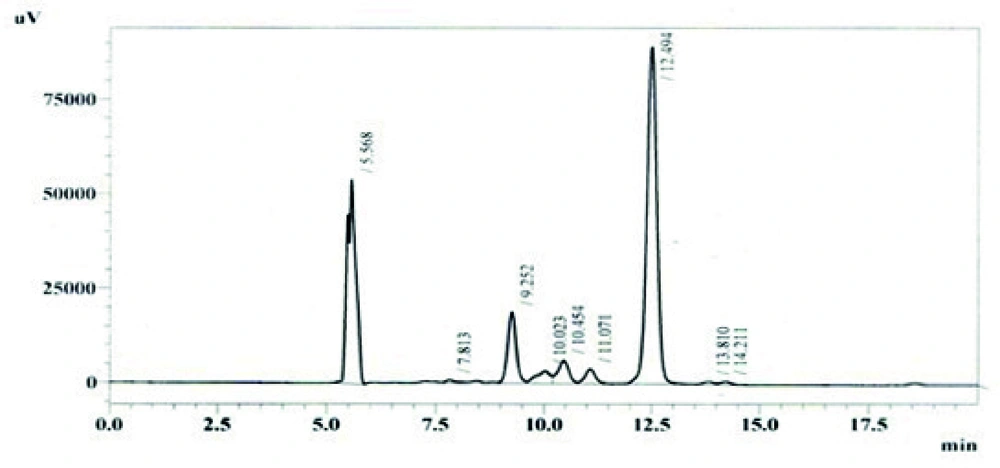
Out of eight different growth media, SM2 was found to be the best medium as it gave the maximum production 17.7798 mg/L of B1b component of avermectin estimated by HPLC as shown in Figure 4. Other media that showed the avermectin B1b production were M1 and M3. The production of avermectin B1b in these media was 12.8118 mg/l and 13.6936 mg/L respectively (results not shown).
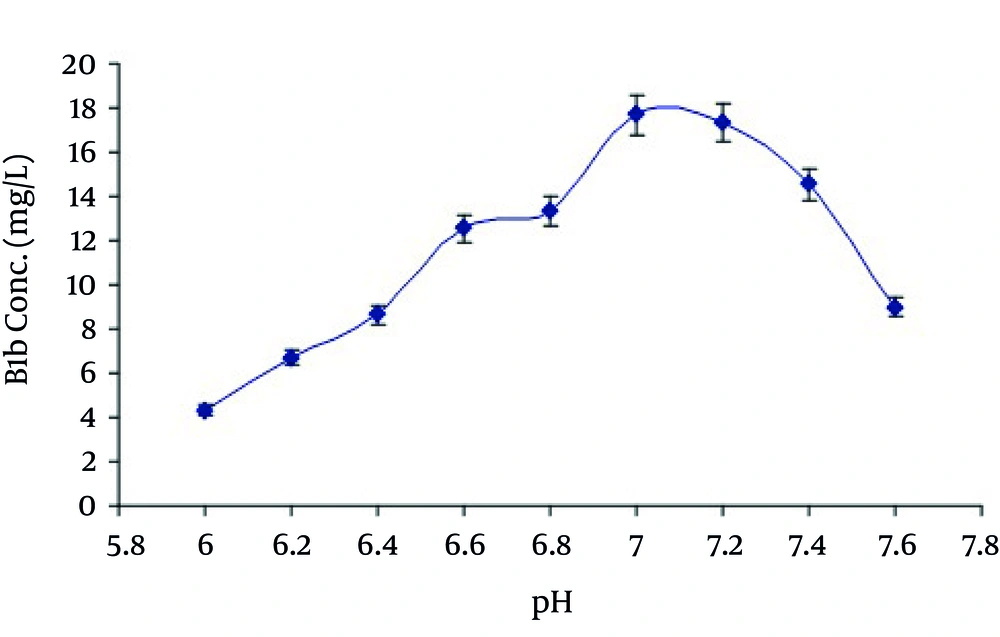
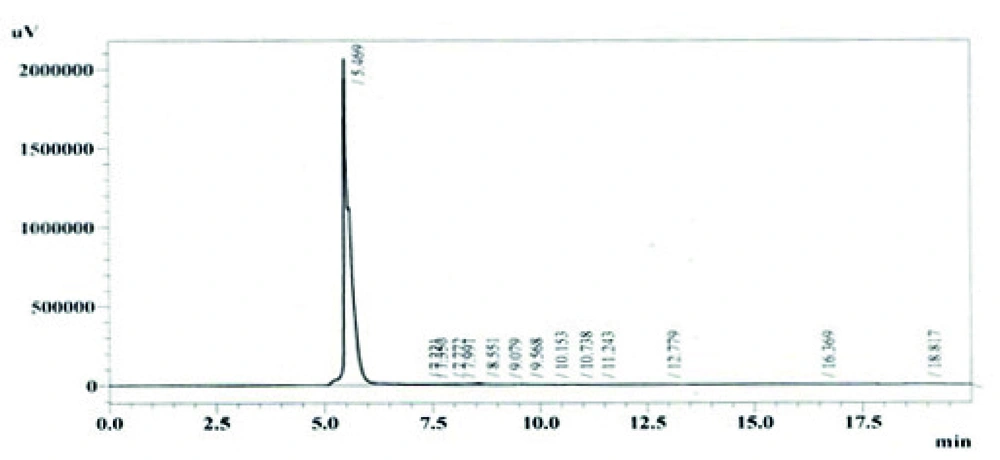
Among other parameters studied, it was revealed from the results that maximum production of avermectin B1b was obtained after 10 days of fermentation at medium pH 7.0, using 10% inoculum medium. The most appropriate incubation temperature for maximum production was 31°C. The HPLC chromatogram for standard solution of abamectin and extracted avermectin B1b are shown in Figures 6 and 7 respectively.
5. Discussion
The selection of a suitable medium plays a vital role to make any fermentation process cost effective. Therefore the present study was conducted to find out the most appropriate medium for the production of avermectin B1b from S. avermitilis DSMZ 41445 by submerged fermentation.
Lazim et.al reported that nitrogen source is very important for the secondary metabolite production (22).
In the present study the media SM1, SM3, SM4, M2 and M4 did not produce any of the avermectin components as shown in Figure 1. Absence of avermectin production in media SM1, SM3 and SM4 may be due the reason that these did not have the main nitrogen source. Also the concentration and nature of nitrogen source greatly influence the production of antibiotics. Antibiotic production is greatly inhibited in a medium containing rapidly utilized nitrogen source. The production of desired secondary metabolite can be achieved by using appropriate nitrogen source in appropriate concentration relative to carbon source (23).
The formation of cell biomass and secondary metabolite formation is also greatly influenced by the rate at which the carbon source is metabolized by the microorganism (24). Productivity of secondary metabolites is lowered due the increase in the concentration of sugars that metabolized rapidly. The SM1, SM3, M2 and M6 have glucose as the carbon source instead of soluble corn starch. Glucose is one of the carbon sources that metabolized very rapidly and it suppresses the production of secondary metabolite formation. It inhibits the formation of most important enzyme in biosynthetic pathway (25).
In the present study the selected medium contained soluble corn starch as carbon source, it degraded very slowly to glucose by the action of α-amylase and glucose remains available at late stages for the production of avermectin B1b. Glucose is required by S.avermitilis for maintenance purpose. In the fermentation process glucose is converted to the oleandrosyl diphosphonucleotide which is used for the biosynthesis of avermectin as an important intermediate (26, 27).
The selected medium SM2 was then further optimized for the maximum production of avermectin B1b from the given strain by studying the production at various process parameters including inoculum size, incubation time, incubation temperature and pH. It is reported that about 310 mg/L avermectin B1a has been obtained when the fermentation of S. avermitilis was done for fermentation period of 5 days at temperature 28°C along with 10% inoculum size and a pH of 7.0 ± 0.2 (19). Fermentation period of 5 days was also employed during the production of avermectin B1a from S.avermitilis by gene replacement (6). About 1.5 folds increase in production of avermectin B1a has been reported from S.avermitilis 14-12A through fermentation when carried out at a temperature 28°C and inoculum size 5%. Selective production of avermectin B components (505 µg/mL) was also obtained by studying the fermentation for 10 days at 28-30oC temperature and the inoculum size of 5% (21, 28).
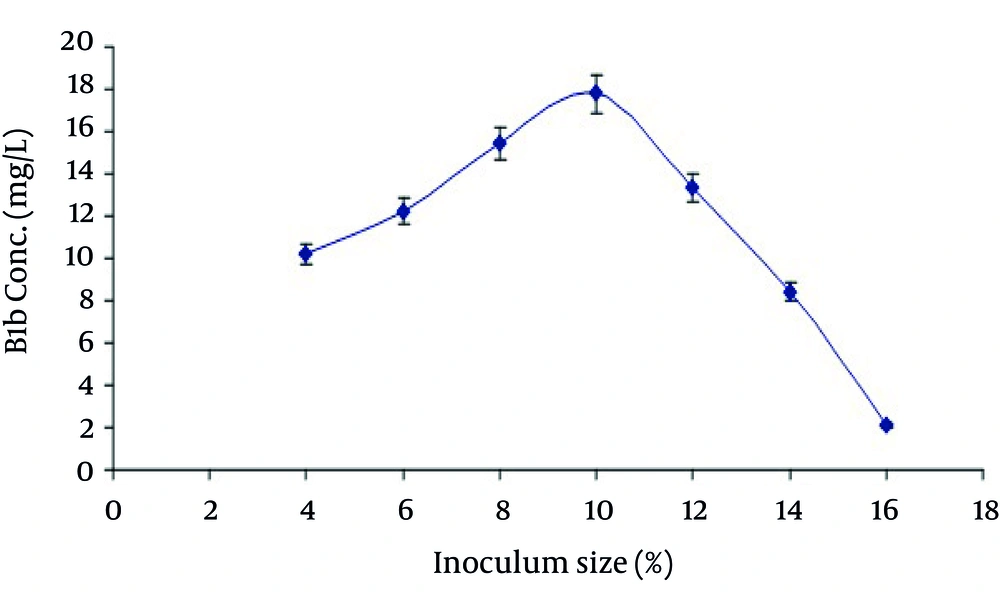
The results of present study revealed that production of B1b was increased with increasing the inoculum size as shown in Figure 2. The maximum production was obtained at inoculum size of 10% v/v and after that it began to decrease. This might be due to the reason that by increasing the inoculum size more than the optimum the nutrient requirements of S. avermitilis cells increased rapidly which resultantly effects the avermectin B1b production. Production of 28 mg/L avermectin B1 (B1a and B1b) from S. avermitilis ATCC 31267 has been reported by employing 10% inoculum size at temperature 30°C with fermentation period of 9 days ( 29 ).
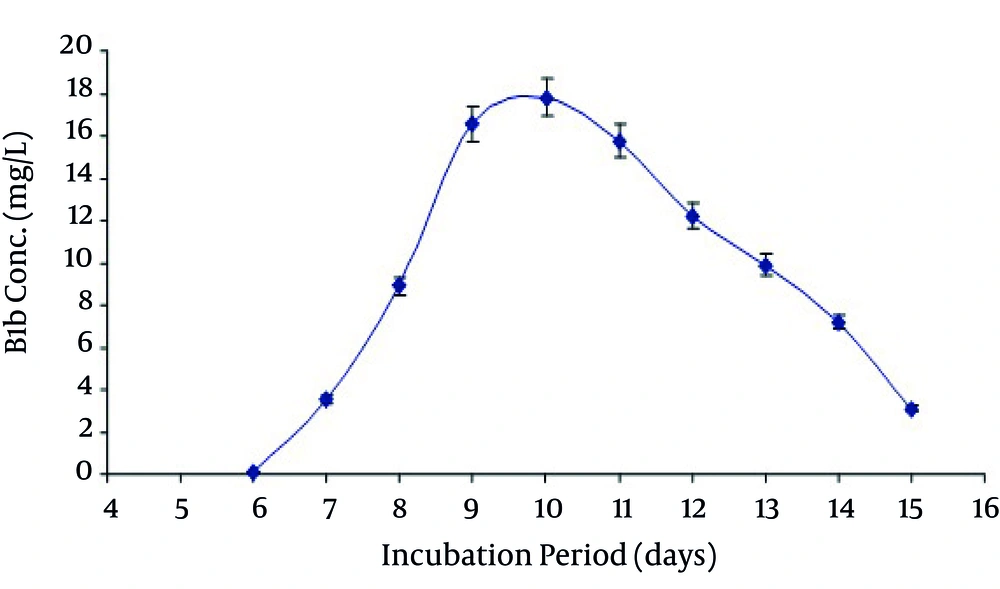
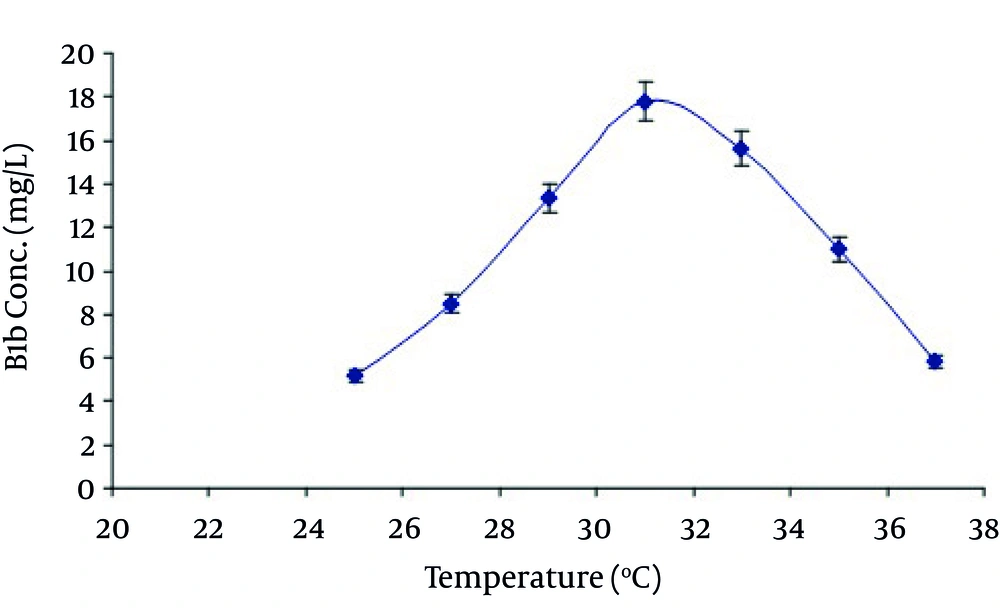
In the present study the avermectin B1b production was also increased by increasing the time of incubation in growth medium. Maximum production was obtained at day 10 th of fermentation. After that it began to decrease as shown in Figure 3. It may be due to the consumption of maximum quantity of soluble corn starch after the action of α-amylase at 10 th day. Production of avermectin B1b from the selected medium was when studied at various temperatures and pH ranging from 25-37°C and 6-7.5 respectively reveled that maximum production was obtained at temperature 31°C at pH 7.0 as shown in Figure 4 and Figure 5.
Inoculum size of 0.03% has also been reported for the production of avermectin (30). About 3321 U/mL of desired avermectin was produced using this condition. S.avermitilis NRRL 8165 had also been reported to produce 17.5 mg/L of avermectin during fermentation of 14 days at temperature 28°C. Inoculum size of 2% and pH of 7.3 ± 2 were maintained during the process (31). Avermectin B1a (2400 ± 200 mg/L) has been obtained with medium pH 7.5±1 and incubation temperature of 28-30°C (32).
The results of the present study indicated that the various compositions of growth medium directly affect the production of avermectin B1b from S. avermitilis 41445. The maximum production of avermectin B1b was obtained by using SM2 as growth medium. Soluble corn starch proved to be the best carbon source. Inoculum size and incubation period also played a vital role in the yield improvement process. Highest level of avermectin B1b was obtained at inoculum size 10% (v/v) and 10th day of incubation period using SM2 medium. Optimized temperature and pH for avermectin B1b production from S.avermitilis 41445 in SM2 medium were 31°C and 7.0 respectively.