1. Background
Acinetobacter baumannii is currently considered to be one of the most important Gram-negative bacteria causing infections in immunecompromised patients, mainly hospitalized in intensive care units (1). A. baumannii is considered to be responsible for 2 - 10% of all Gram-negative bacterial infections of patients in intensive care units in Europe and the United States (2).
Infection due to A. baumannii has become a significant challenge for healthcare systems. Several associated mechanisms with carbapenem resistance have been described in A. baumannii, including metallo B-lactamases (MBLs), carbapenem-hydrolysing oxacillinases, decreased permeability due to the loss of outer membrane proteins (OMPs), overexpression of efflux pumps and hyperproduction of AmpC B-lactamases (3). The terms pan drug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) are explained, respectively, resistance of a pathogen to all, resistance to all expect 1 or 2, and resistance to ≥ 3 classes of antimicrobial agents (4).
2. Objectives
The purpose of the present study was to investigate antibiotic susceptibility profile and the rate of XDR or PDR A. baumannii isolated from clinical and environmental specimens of intensive care units of central teaching hospital of Arak University located in center of Iran.
3. Patients and Methods
The outbreak occurred in the 350-bed central teaching hospital of Arak University of Medical Sciences in the central part of Iran and a total of 63 non-duplicate A. baumannii isolates were collected from clinical and environmental specimens from March to September 2011. Clinical samples consisted of cerebrospinal fluids (n = 3, 5.4%), surgical wound swabs (n = 4, 7.2%), urine samples (n = 6, 10.7%), blood samples (n = 12, 21.4%), respiratory secretions (n = 31, 55.3%) obtained in surgery, neurology and neurosurgery intensive care unit (ICU) wards. Environmental samples of these ICU’s were obtained from ventilator tubing (three isolates), suction catheters (two isolates), patient’s mattress (one isolate) and the barometer (one isolate). The research was approved by the Ethics Committee of the Faculty of Medicine at the Arak University of Medical Sciences (633).
A. baumannii was identified by Gram staining, colony morphology, motility, cytochrome oxidase reaction, standard biochemical tests and its growth at 43°C, as well as Microgen kit (Microgen Bioproducts, UK). In addition, phenotypic characterization of A. baumannii was confirmed by 16S-rRNA intergenic spacer sequencing (ITS) and OXA-51 PCR ( 5 ). The primers and programs can be found in Table 1.
Susceptibilities of the bacterial isolates to a 23 different antimicrobial agents (Mast, UK) were defined by using the standard disk diffusion method according to Clinical and Laboratory Standard Institute (CLSI-2011) guidelines and European Committee on Antimicrobial Susceptibility Testing (EUCAST-2011) instructions (Table 2). Susceptibility to Colistin was tested by disk diffusion method using 10 μg Colistin sulfate disks. Isolates were considered sensitive if the inhibition zone was 11 mm or more ( 7 ). The results were verified by E-test (bioMérieux, Marcy l’Etoile, France) on Imipenem and Colistin according to CLSI-2011 guidelines. Intermediate sensitivity was considered as resistance.
The modified Hodge test (MHT) as recommended by CLSI was used for isolates which showed intermediate or susceptible zones to Imipenem in disk diffusion (8 ). All isolates were detected for Metallo-β-lactamases (MBL) production by the E-test MBL strip method; a reduction in the minimum inhibitory concentration (MIC) of Imipenem in the presence of ethylene diamine tetra-acetic acid (EDTA) more than or equal to eight-fold was an indicator of MBL activity (Figure 1) (3 , 9 ). In addition, all isolates were evaluated by AmpC disk test for detection of AmpC betalactamase resistant (Figure 2) (10 , 11 ).
| Antibiotics | Clinical Isolates, No. (%)( n = 56) | Environmental Isolates, No. (%) (n= 7 ) |
|---|---|---|
| Aztreonam | 56 (100) | 7 (100) |
| Piperacillin | 56 (100) | 7 (100) |
| Piperacillin/tazobactam | 56 (100) | 7 (100) |
| Amoxicillin/Clavulanic acid | 56 (100) | 7 (100) |
| Cefoxitin | 56 (100) | 7 (100) |
| Cefotaxime | 56 (100) | 7 (100) |
| Ceftazidime | 56 (100) | 7 (100) |
| Cefepime | 56 (100) | 7 (100) |
| Ciprofloxacin | 56 (100) | 7 (100) |
| Levofloxacin | 56 (100) | 7 (100) |
| Erythromycin | 56 (100) | 7 (100) |
| Clindamaycin | 56 (100) | 7 (100) |
| Rifampin | 56 (100) | 7 (100) |
| Chlorafenicol | 56 (100) | 7 (100) |
| Trimethoprim/Sulfamethoxazole | 56 (100) | 7 (100) |
| Netilmicin | 31 (56) | 3 (43) |
| Tetracycline | 50 (90) | 6 (86) |
| Amikacin | 43 (77) | 7 (100) |
| Colistin | 4 (7) | 3 (43) |
| Tigecycline a | 55 (98) | 7 (100) |
| Meropenem | 48 (86) | 5 (72) |
| Imipenem | 48 (86) | 5 (72) |
| Gentamicin | 50 (90) | 6 (86) |
aBritish (BSAC) tigecycline susceptibility breakpoints (12)
4. Results
The antimicrobial susceptibility profiles of 63 isolates are shown in Table 2, describing elevated levels of A. baumannii resistance, which pose serious treatment challenges. All isolates were resistant to Cefoxitin, Cefotaxime, Ceftazidime, Cefepime, Piperacillin, Piperacillin/Tazobactam, Amoxicillin/Clavulanic acid, Aztreonam, Ciprofloxacin, Levofloxacin, Erythromycin, Clindamaycin, Chlorafenicol, Rifampin and Trimethoprim/ Sulfamethoxazole. High rates of resistance to Imipenem (84%), Meropenem (84%), Gentamicin (89%), Amikacin (80%) and Netilmicin (54%) were observed. Colistin had good antimicrobial activity (89%).
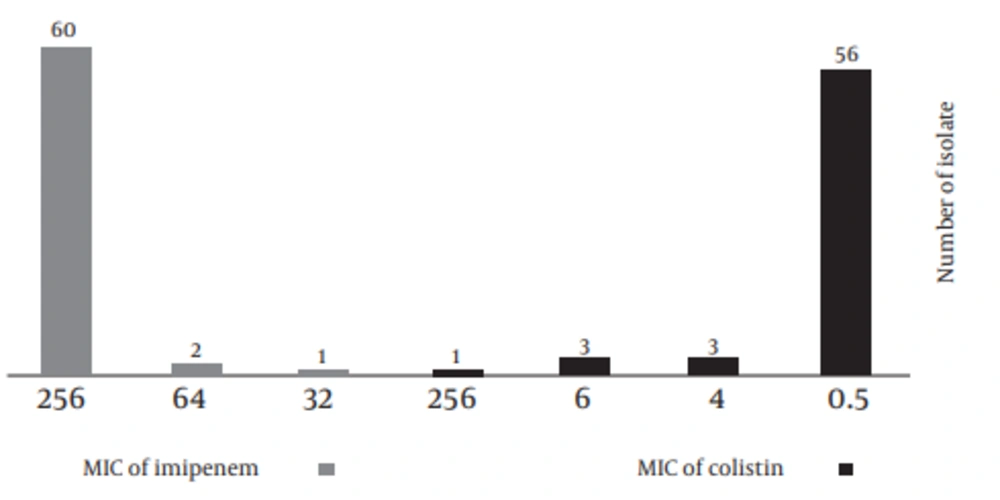
Of 56 clinical and seven environmental isolates, eight isolates showed intermediate and two isolates revealed susceptible zone to Imipenem, using disk diffusion method. Out of these 8 and 2 isolates, 3 and 1 isolates were positive in the MHT test, respectively. Among all of the A. baumannii isolates, 45 (71.4%) were AmpC β-lactamase producers and 47 (75%) were MBL producers analyzed by MBL E-test. The prevalence of PDR and XDR among our isolates was found to be 11% and 89%. MIC of Colistin and Imipenem are presented in Figure 3.
5. Discussion
Based on these findings, to treat A. baumannii in our hospital, third- and fourth-generation of Cephalosporins are no longer secure. B-lactamase-resistant agents including Piperacillin/Tazobactam and Amoxicillin/Clavulanic acid are not proper choices for treating A. baumannii infections; the Monobactam Aztreonam is an almost ineffective antibiotic. Moreover, A. baumannii isolates showed a high level of resistance to Tigecycline and Quinolones (98% to 100%) which are due to over-expression of AdeABC efflux pumps and mutation in the Quinolone resistance-determining region (QRDR) of gyrA and parC genes, respectively (2).
Colistin is only effective against 89% of A. baumannii isolates. Unfortunately, our knowledge on the pharmacokinetics and pharmacodynamics of Colistin are limited, and the current dosage regimens used clinically are based on experience acquired during the past 30 years (13).
In current study, emergence of heterogeneous resistance was confirmed in three strains through E-test and disk diffusion methods for Colistin, isolates presenting colonies inside the inhibition zone were considered to have phenotypic heterogeneous resistance to Colistin (14). Isolated strains are increasingly becoming pan resistant and even those strains that are especially resistant are difficult to treat due to the nephrotoxicity and increasing intermediate sensitivity to Colistin.
In the current study, 53 isolates were identified as Imipenem resistant by disk diffusion method, whereas all clinical and environmental A. baumannii isolates were resistant to Imipenem tested by E-test method, according to CLSI and EUCAST guidelines. Other studies in Asian countries have shown a wide range of A. baumannii Imipenem–resistance in this region including 49% in Iran, 65% in Syria and 45%-90% in Saudi Arabia (15-17). The presence of six MHT negative isolates, indicating that other mechanism of Carbapenem resistant such as metallo-beta-lactamase and AmpC β-lactamase have also contributed to Carbapenem resistance among these isolates. Out of these six isolates, four were MBL, AmpC producer and two of the other isolates shown to be AmpC positive and have MBL negative pattern.
MBL was produced by 47 (75%) and AmpC β-lactamase by 45 (71.4%) of the Imipenem resistant A. baumannii. Therefore, AmpC β-lactamase and MBL could be an important contributory factor for Imipenem resistance among the isolates in our hospital (11). Modified Hodge test may not be a useful screening test for Carbapenemases as some of MBL producing isolates were not detected by this test (11, 18).
The selective pressure caused by indiscriminate usage of broad-spectrum antibiotics in empirical therapy of hospital infections and environmental contamination are the main reason for such an increased number of patient morbidity and mortality that is related to the infections with the mentioned microorganisms. The MBL, AmpC β-lactamase and combination of MBL and AmpC production in PDR, XDR A. baumannii isolates are the main reasons of Carbapenem resistance.