1. Background
Because pathogenic fungi have become resistant to current antifungal agents, the development of new antibiotics or novel antimicrobial formulations is essential. The antifungal capacities of various organic and inorganic materials against pathogenic fungi have been tested in recent decades and the development of such novel compounds is ongoing (1). Selenium (Se) is a micronutrient metalloid incorporated (in the form of selenomethionine or selenocysteine) in the structure of enzymes such as glutathione peroxidases, iodothyronine deiodinases, and thioredoxin reductase, which are involved in antioxidant defense, detoxification, and metabolism, respectively (2, 3). The low toxicity of Se nanoparticles (NPs) and properties such as antibacterial, antiviral, and antioxidant activities have attracted research attention (4, 5).
Nanotechnology allows the fabrication of novel compounds (i.e., NPs or nanoconjugates) with novel biological properties (6-9). Physicochemical techniques (10, 11), biological methods, and the synthesis of nanostructures with bacterial and fungal strains as well as several plant extracts offer novel, clean, non-toxic, and eco-friendly methods for the production of Se NPs (12, 13).
Although Se compounds such as selenium sulfide (SeS2) have long been used as antifungal agents (14), the antifungal activity of biogenic Se NPs against Aspergillus fumigatus and Candida albicans has not been investigated. In the present study, biogenic Se NPs were purified from the whole-cell lysate of Bacillus sp. MSh-1 and characterized. Then, the antifungal efficacy of these novel NPs against A. fumigatus and C. albicans was studied.
2. Objectives
The aim of this study was to synthesize Se NPs, test them against the yeast cells of C. albicans and mold cells of A. fumigatus, and compare their minimum inhibitory concentrations (MICs) to determine their sensitivities.
3. Materials and Methods
3.1. Chemicals
Selenium dioxide (SeO2), nutrient broth, potato dextrose agar, Sabouraud’s dextrose agar medium, 1-octanol, sodium dodecyl sulfate, and Tris-base were purchased from Merck Chemicals (Darmstadt, Germany). All other chemicals and solvents were of analytical grade.
3.2. Preparation of Biogenic Se NPs
Se NPs were prepared by reducing Se+4 ions with the Bacillus sp. MSh-1, which was previously identified with a 16S ribosomal DNA method (GenBank accession number: GU183144.1) (13). Briefly, sterile nutrient broth was supplemented with SeO2 (1.26 mM) and inoculated with an overnight colony of Bacillus sp. MSh-1 in a shaker incubator (30°C, 150 rpm) for 14 hours. Subsequently, cells containing elemental Se were isolated from the medium via centrifugation (4000 × g, 10 min). Cells were disrupted by using a mortar and pestle after washing with sterile normal saline and freezing with liquid nitrogen. One milliliter of disrupted cells containing Se NPs was dispersed in 2 mL 1-octanol, and the resulting two-phase system was centrifuged (5000 × g, 10 min). The biogenic Se NPs were settled and washed with chloroform, ethyl alcohol, and distilled water (13).
3.3. Characterization of Se NPs
After the Se NPs were sonicated (100 W, 5 minutes), transmission electron microscopy (TEM) images were recorded with a (Zeiss Supra 55 VP, Jena, Germany) equipped with an energy-dispersive X-ray (EDX) microanalyzer at an accelerating voltage of 100 kV. The size distribution pattern of the Se NPs was plotted by manually counting 400 individual particles from various TEM images.
3.4. Antifungal Activity
The antifungal activities of Se NPs against A. fumigatus (ATCC 5662) and C. albicans (ATCC 4862) were determined by using the National Committee for Laboratory Standards (CSLI) for yeasts (CSLI M27-A) and filamentous fungi (CSLI M38-P) approved for both macro- and microdilution methods (15, 16). A. fumigatus and C. albicans were cultured at 25°C in potato dextrose agar and Sabouraud’s dextrose agar medium, respectively. Preparations of 0.5 - 2.5 × 103 and 2 - 5 × 104 conidia/mL (final concentration) were obtained from A. fumigates and C. albicans, respectively, by using a standard neobar slide with the tubes containing 3 mL of sterile saline solution. RPMI 1640 medium containing l-glutamine and phenol red was buffered to pH 7.0 at 25°C with 0.165 mol/L 4-morpholinepropanesulfonic acid and used as the MIC method. Se NPs were suspended in sterile distilled water and subsequently diluted to a final concentration of 10 - 200 µg/mL with the medium according to the CLSI standard. The MICs of Se NPs capable of fungal growth inhibition after 48 hours of incubation at 37°C were determined. RPMI medium containing fungal suspension without Se NPs was used as negative control. Amphotericin B (AmB) (10 μg/mL) and nystatin (15 μg/mL) were used in the same medium as positive controls for A. fumigates and C. albicans, respectively. All antifungal experiments were repeated five times.
4. Results
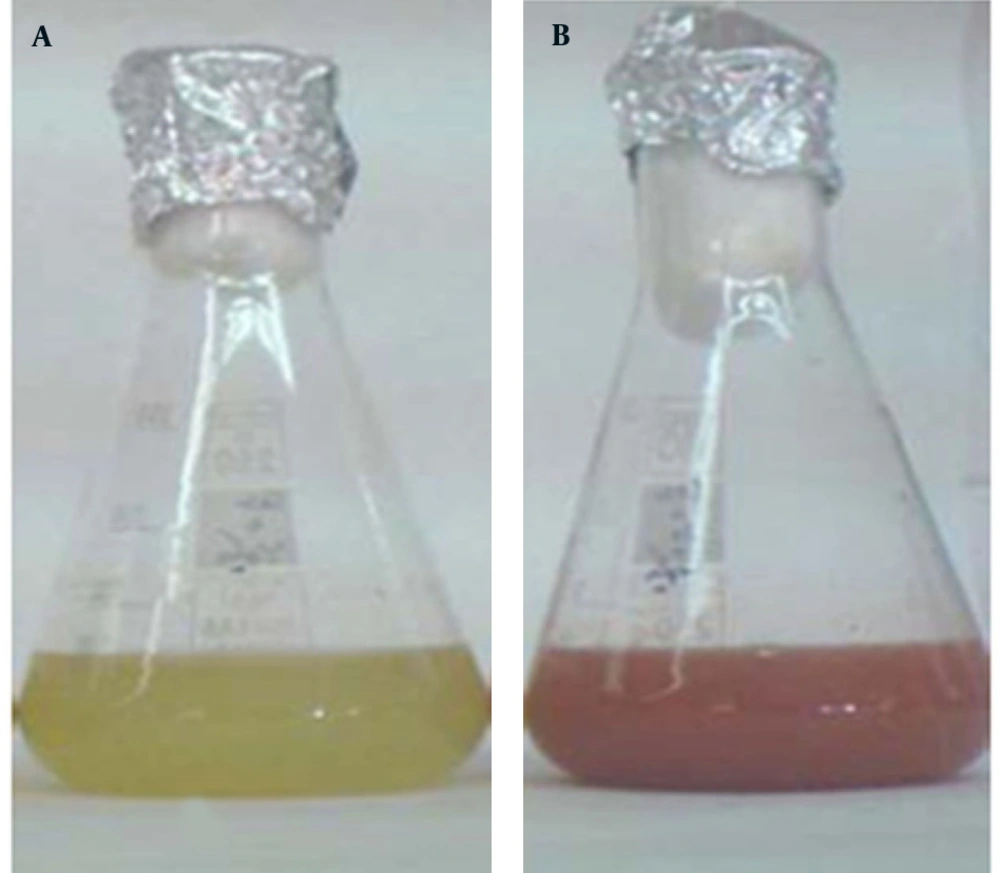
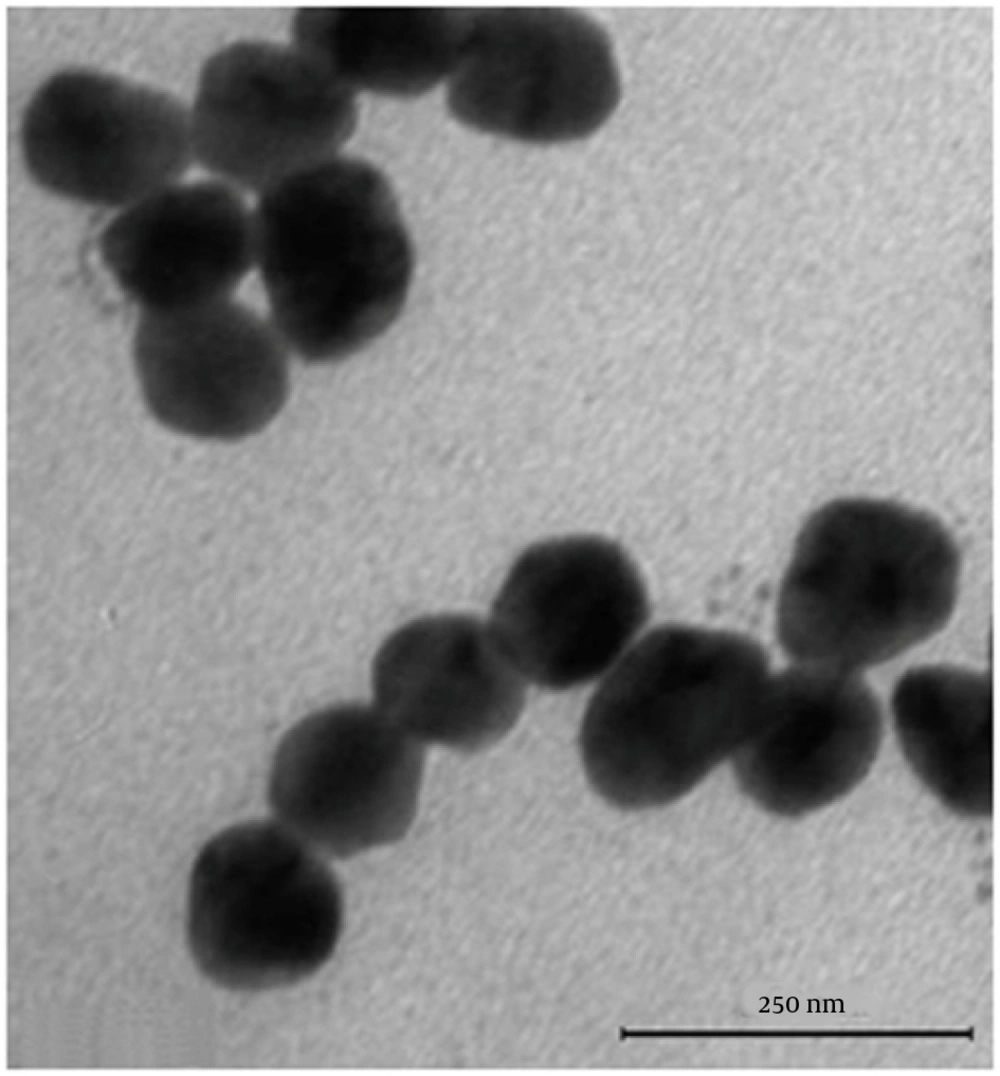
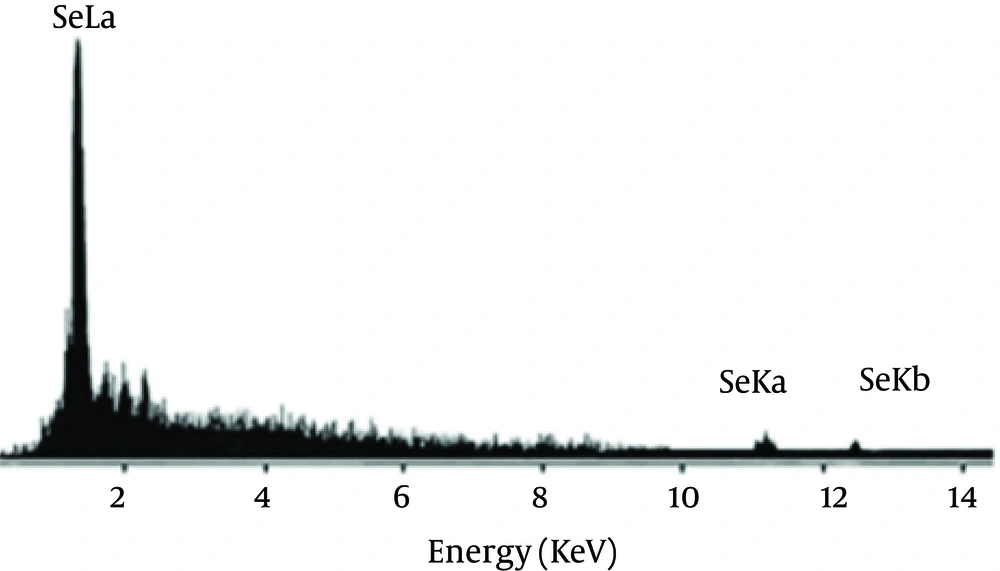
Se NPs were successfully synthesized with Bacillus sp. MSh-1 as evidenced by a significant change in the cultivation medium from colorless to insoluble orange-red, indicating the presence of elemental Se (Se0), after 14 hours (Figure 1). The TEM images of the Se NPs (Figure 2) showed that these well-dispersed spherical nanostructures had diameters ranging from 80 to 220 nm. The EDX pattern of the biogenic NPs had a strong SeLα peak at 1.37 KeV (Figure 3). Furthermore, SeKα and SeKβ peaks were observed at 1.37 and 11.22 KeV, respectively (see Figure 3). Subsequently, no signals for other elements were observed in the EDX pattern, which showed only the presence of the aforementioned signals for Se atoms (with the weight percent equal to 100).
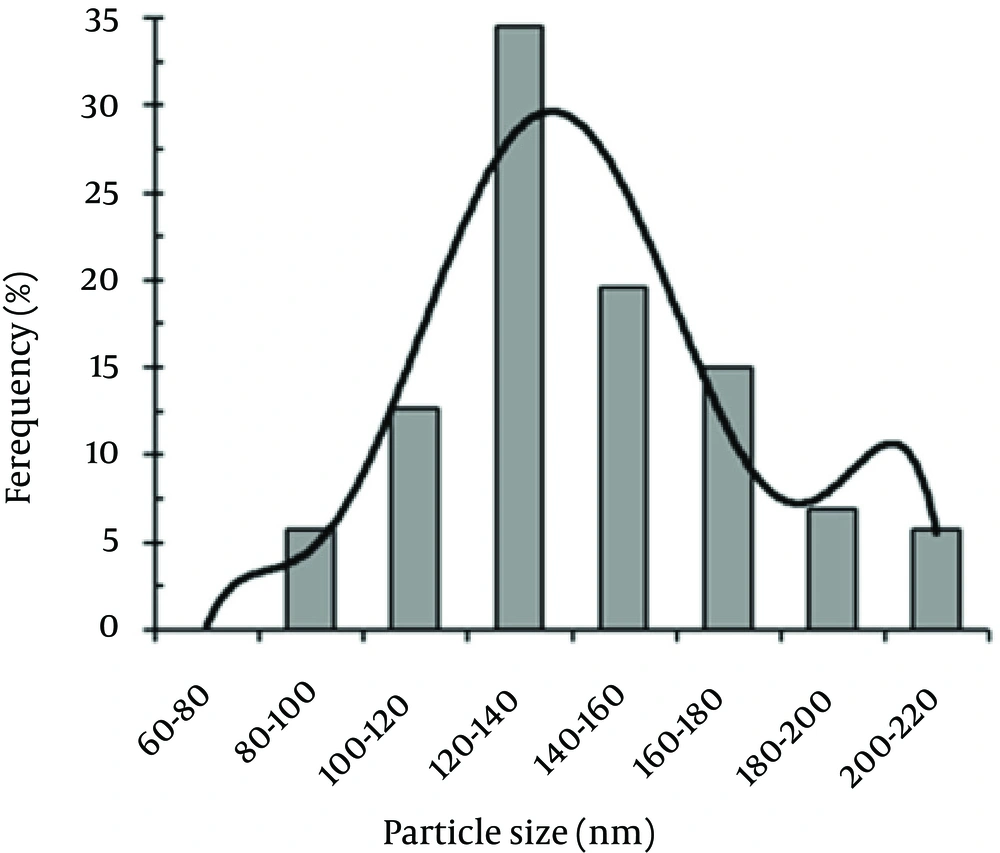
The size distribution pattern revealed that 120- to 140-nm NPs were the most common particles (Figure 4). The antifungal activities of the Se NPs against A. fumigatus and C. albicans are shown in Table 1. The measured MICs for A. fumigatus (100 μg/mL) and C. albicans (70 μg/mL) showed that the biogenic Se NPs had good anti-fungal activity.
| Fungal Strain | Antifungal Activity of Se NPs MIC a | Active Antifungal Drug MIC |
|---|---|---|
| Aspergillus fumigatus | 100 | Amphotripcin B 1 |
| Candida albicans | 70 | Nystatin 0.05 |
a MIC, minimum inhibitory concentration.
5. Discussion
Because nystatin resistance in Candida sp. has been reported, especially in immunocompromised patients, and amphotericin B is still widely used as a first-line drug for the treatment of invasive aspergillosis despite its low efficacy, the development of new agents that can be used against fungi is critical. Ramamurthy et al. used fenugreek seed extract for the biosynthesis of Se NPs (50 - 150 nm) after 72 hour incubation (17). In another study, supplementation of the culture supernatant of A. terreus with SeO2 (100 μg/mL) produced Se NPs with an average size of 47 nm (18, 19). Bacillusmegaterium (a halophile strain) strongly reduced selenite (up to 0.25 mM) to Se NPs after 40 hours of incubation (17). Our results showed that the Bacillus sp. MSh-1 synthesized Se NPs (80 - 220 nm) after 14 hours of incubation.
The resistances of Candida sp. to nystatin has been reported, especially in immunocompromised patients (20). Amphotericin B is still widely used as first-line drug for treatment of invasive aspergillosis despite its lower efficacy (21, 22). Despite the development of new antifungal drugs, the number and variety of effective treatments remain limited (23). Therefore, use of NPs against pathogenic fungi is yet another novel approach. Se NPs have a variety of biological properties and might be candidates for a range of applications (24-26). Kazempour et al. (27) reported that the MICs of Se NPs (90 - 320 nm) biosynthesized with a two-phase system by using Klebsiella pneumoniae and without purification against A. niger and C. albicans were 250 µg/mL and 2000 µg/mL, respectively. The results of another study of biogenic Se NPs (100 - 600 nm) showed the highest antifungal activity against Malassezia sympodialis (MIC 10 µg/mL) and M. furfur (MIC 50 µg/mL) (28). Furthermore, the measured MICs of nanoselenium against A. niger, A. terreus, A. flavus, A. fumigatus were 260 µg/mL, 60 µg/mL, 220 µg/mL, and 100 µg/mL, respectively, against AmB (28). Although our Se NPs (80 - 220 nm) were smaller than the Se NPs (100 - 600 nm) prepared by using K. pneumoniae, they showed the same levels of antifungal activity against A. fumigatus.
The results of the present study suggest that nanoscale biogenic elemental Se has antifungal activity against A. fumigatus and C. albicans. The mechanism of this antifungal effect is unknown and merits further in vivo and in vitro studies.