1. Background
Sporulation is one of the important properties of Clostridium difficile. The bacterium is disseminated in the anaerobic condition of the colon and environment by the formation of spore and survives for a long time in harsh conditions. The spore is very important for spore-forming bacteria such as C. difficile. Because their infections are transferred by spores (1). Clostridium difficile contains several toxin-producing variants, whether may or may not be associated with the C. difficile antibiotic-associated diarrhea (AAD). These toxin-producing variants consist of C. difficile A+/B+/CDT-, A+/B+/CDT+, A-/B+/CDT-, A-/B+/CDT+, A-/B-/CDT+, A+/B+/CDT-, A+/B-/CDT-, A-/B-/CDT-, and A+/B-/CDT- (2, 3).
The most prevalent type of C. difficile in clinical samples is the A+/B+/CDT- toxin producing type (4). There is another variant, A-/B-/CDT-, which is common in the clinical specimens. Based on the type of sample source, geographical area, age, and detection methods vary from 42% - 50% (5, 6). This non-producing type is prevalent in the stool samples that are collected from environmental samples (30.8%) and hospitalized patients (6.5%) (7), in-patient wards (8.5 - 46%) (8-10), hospitalized infants (63%) (11), in- and out-patient infants (58% - 82%) (12) and in a neonate intensive care unit in Japan (96%) (13). Some researchers have considered them to be pathogenic forms (14), while others consider them to be non-pathogenic (5). Some investigators have mentioned these strains are helpful because common antibodies against non-pathogenic strains (A-/B-/CDT-) protect the individuals against infections with the toxigenic variants (15).
2. Objectives
Since the ability of spore formation must be a key property that helps the non-toxigenic isolates to survive in the nature or clinical samples; therefore, the aim of this study is to evaluate spore production capability of nonpathogenic of C. difficile isolates (A-/B-/CDT-) compared to toxigenic C. difficile (A+/B+/CDT-) in the absence and presence of antibiotic in the laboratory condition.
3. Methods
3.1. Bacterial Isolates
Two non-toxigenic C. difficile isolates (A-/B-/CDT-) and one toxigenic A+/B+/CDT- were isolated from diarrhea samples (16). The C. difficile ATCC 9689 (A+/B+/CDT-) was prepared from the microbial collection of Microbiology Department of Kerman University of Medical Sciences. All isolates were confirmed by latex agglutination test as C. difficile. The existence of toxin genes and the ability of toxin production were confirmed by the PCR method and commercially ELISA technique, respectively (17-20). In brief, the isolates were cultured on brain heart infusion (BHI) blood (5% defibrinated sheep blood) agar at 37 ºC for 24 hours in an anaerobic jar (Merck, Germany). The DNA was extracted from them by commercially kit (CinnaGen, Iran). The existence of tcdA and tcdB genes was carried by PCR method according to the previous study (20). For the ability of toxin production, the isolates were cultured in the pre-reduced medium [BHI broth (GIBCO, Scotland) supplemented with 1% yeast extract (BBL, USA) and 0.5% L-cysteine (Merck, Germany)] for 48 hours in the anaerobic jar. The supernatants were separated by centrifuge and used for toxin production evaluation. The ability of toxin production was performed by C. difficile A & B ELISA kit (tgc BioMic, Germany) according to the kit’s guidelines (21). This kit detects toxins (A & B) in supernatant simultaneously.
3.2. Minimum Inhibitory Concentration Determination
Minimum inhibitory concentration (MIC) of three clinical isolates and ATCC 9689 strains was performed in triplicates for (ceftazidime) CAZ, (clindamycin) CLI and (vancomycin) VAN (all Sigma, USA) by the microdilution method (22). In brief, the isolates were cultured on BHI agar supplemented with 5% defibrinated sheep blood and incubated in the anaerobic jar for 18 hours. The bacterial suspension was prepared upon 0.5 McFarland (1.5 × 108 CFU/mL) and used for inoculation to the tube containing pre-reduced medium (PRM) (control and ½ × MIC of VAN, CLI, and CAZ). Micro-titer plates containing ½ × MIC antibiotics in pre-reduced medium two-fold serially diluted were inoculated with ~106 colony forming unit (CFU)/mL from fresh bacterial suspension (18-hour culture), and incubated in an anaerobic jar (23). The MIC was recorded after two days (24). The bacterial suspension preparation and inoculation to the tube with PRM were performed less than 30 minutes (25).
3.3. Culturing and Spore counting
Approximately ~106 CFU/mL bacteria from the suspension (1.5 × 108 CFU/mL) of 18-hour culture were inoculated to the 5 mL tubes (controls and each of the antibiotics) and incubated in anaerobic condition for 24, 48, and 96 hours at 37°C (22). At the appropriate times (24, 48, and 96 hours), one milliliter of the cultures (control medium and the media containing ½ × MIC) was frozen at 70°C. After one month the frozen cultures were thawed at room temperature and heated 10 minutes at 80°C to kill the vegetative form and for the spores to become pre-activated (26). After preparation of serial dilution, 100 μL of the heated cultures were inoculated to Columbia blood agar (BBL, USA) supplemented with 5% defibrinated sheep blood. The plates were incubated under anaerobic jar for 72 hours at 37ºC. According to the investigation of Aldape and colleagues, the number of viable spores was counted (27). For reducing the toxic effects of oxygen, all stages were performed in less than 15 minutes (28). All experiments were performed in triplicate.
4. Results
4.1. Minimum Inhibitory Concentration
The MIC of C. difficile ATCC 9689 strain and clinical isolates (toxigenic and non-toxigenic) isolates are shown in Table 1. All isolates and ATCC 9689 were sensitive to VAN, CLI, and CAZ (23, 29).
| Clostridium difficile | Toxigenic C. difficile | Non-Toxigenic C. difficile | ||
|---|---|---|---|---|
| Isolate source | ATCC 9689 | Clinical | Clinical 1 | Clinical 2 |
| Toxin production type | A+/B+/CDT- | A+/B+/CDT- | A-/B-/CDT- | A-/B-/CDT- |
| MIC, μg/mL | ||||
| VAN | 0.5 ± 0 | 0.5 ± 0 | 0.84 ± 0.29 | 0.42 ± 0.14 |
| CLI | 1 ± 0 | 2 ± 0 | 3.4 ± 1.1 | 2 ± 0 |
| CAZ | 13.4 ± 4.6 | 8 ± 0 | 2 ± 0 | 6.7 ± 2.3 |
| ½ × MICa, μg/mL | ||||
| VAN | 0.25 | 0.25 | 0.5 | 0.25 |
| CLI | 0.5 | 1 | 2 | 1 |
| CAZ | 8 | 4 | 1 | 4 |
MIC and ½ × MIC of Clinical Isolates and ATCC 9689 Strain for VAN, CLI, and CAZ
4.2. Control
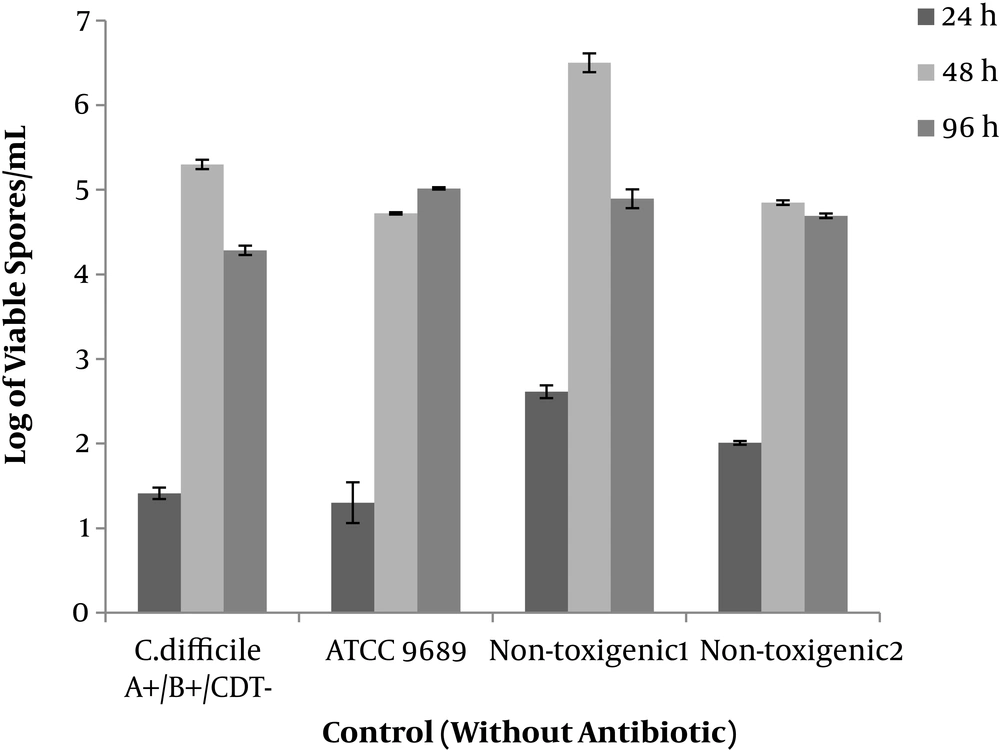
Two clinical toxin non-producing types of C. difficile isolates and two toxin producing types (ATCC 9689 and a clinical isolate) started spore production within 24 hours and reached the maximum level at 48 hours. The maximum level of spore production was 6.5 Log/mL in the case of non-toxigenic isolate. In total toxigenic and the non-toxigenic isolates had a similar pattern in spore production at different times (Figure 1).
4.3. Vancomycin, Clindamycin and Ceftazidime
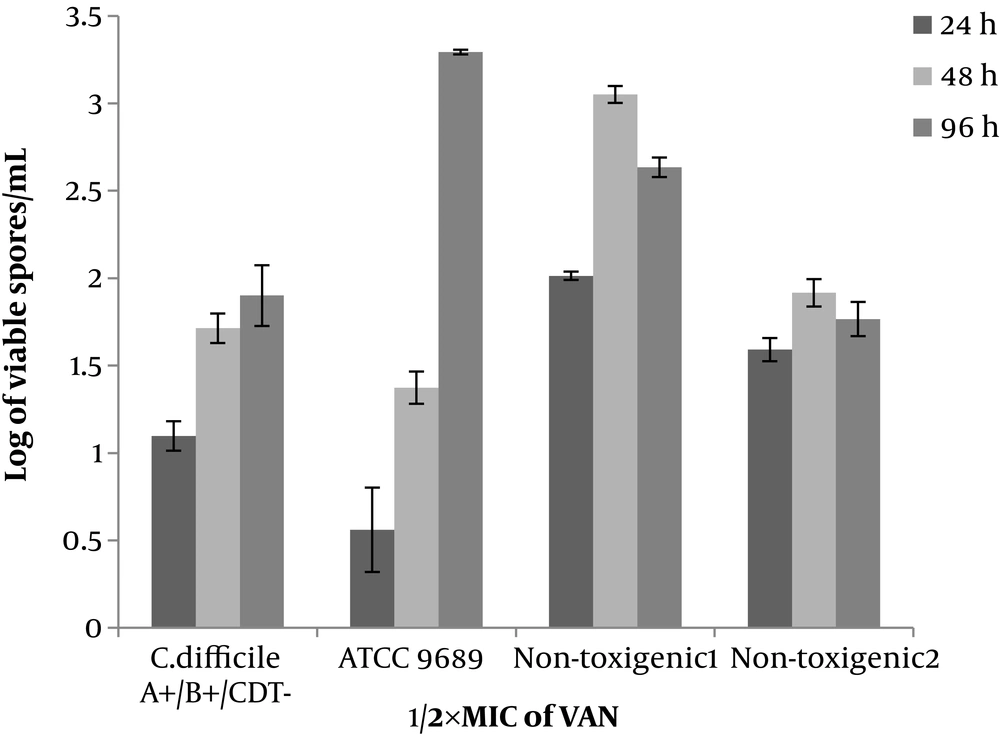
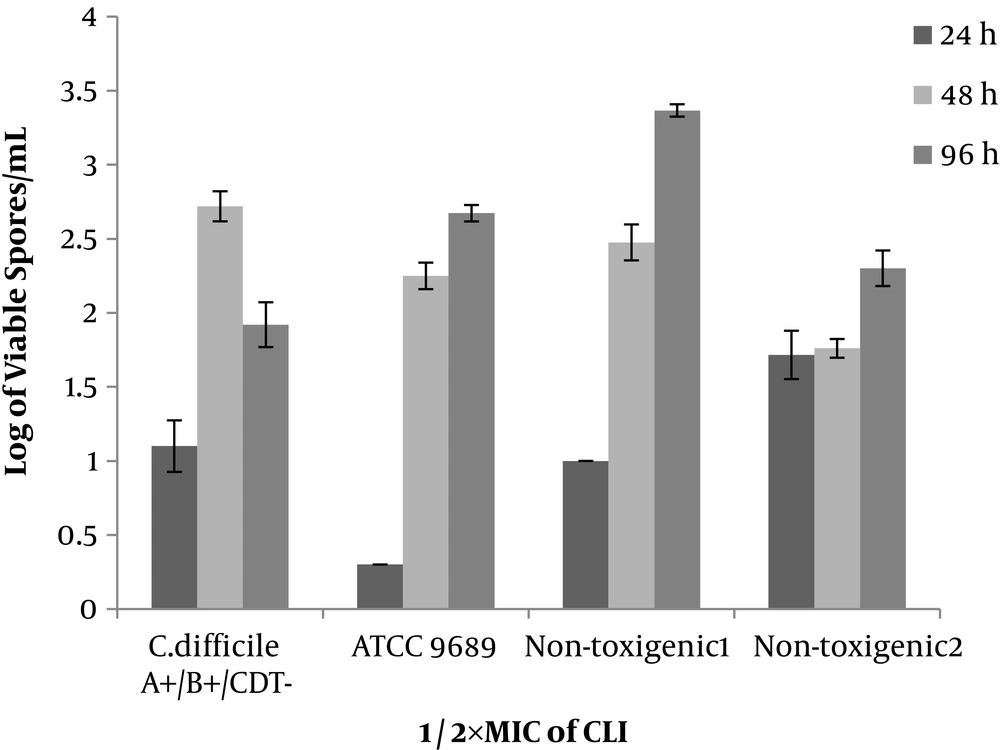
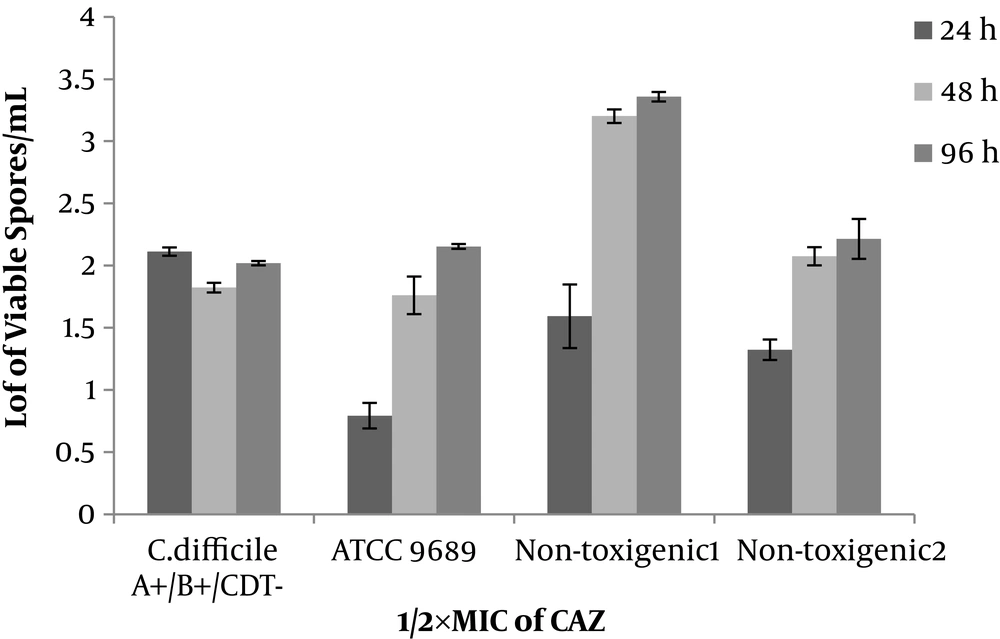
Non-toxigenic isolates similar to toxigenic ones (ATCC 9689 strain and clinical isolate) in the media (with ½×MIC of antibiotics) started the spore production within 24 hours and continued it until 48-hour incubation. In addition, the spore production of ATCC 9689 strain did not stop at 48 hours, while continued and reached 4.3 to 6.5 Log/mL after 48-96-hour incubation. In the ½×MIC of antibiotic (VAN, CLI, and CAZ), the pattern of the spore production was similar in toxigenic and non-toxigenic isolates. The number of produced spores was 1.7 - 3.2, 2.3 - 3.3, and 2 - 3.5 for VAN, CLI, and CAZ, respectively. Generally, VAN, CLI, and CAZ inhibited the spore production in toxigenic and non-toxigenic isolates (Figures 2-4). All isolates (either toxigenic or non-toxigenic strains) and ATCC 9689 strain continued to spore production until 96 hours in the media containing ½ MIC of VAN, CLI, and CAZ. While in the control, media the number of formed spores was similar to 48-hour-periods or sometimes less than 48 hours (Figures 1-4).
5. Discussion
Because of deletion, insertion, and point mutations in the regulatory genes and pathogenicity loci, non-toxigenic isolates of C. difficile have lost the capability of toxin production (3, 30). However, it is noted that non-toxigenic strains protect the individual against the infection of toxigenic strains (31). The previous studies have mostly insisted on the investigation of spore production in the virulent and hypervirulent strains such as C. difficile ribotype 02, 014, 018, and 027 (32, 33). The investigation in different aspects showed the sporulation is an intrinsic and genetic property and it is related to the type of C. difficile (33, 34). The isolates of C. difficile with a high power of sporulation are more virulent and are more related to recurrent infection (35). In our study and based on the literature, it seems the non-toxigenic C. difficile isolates produce spore, similar to toxigenic isolates in the free antibiotic medial and probably in their natural environment (colon). They have retained the capability of spore production.
Clostridium difficile is the most antibiotic-associated diarrhea pathogen and produces spores in the natural environment (colon) in the presence the antibiotics (36). Therefore, spore production of C. difficile strain in the presence of antibiotics was performed and it was shown that the antibiotics might or might not inhibit the sporulation. The VAN and fidaxomicin as the treatment choice of C. difficile AAD inhibit sporulation in the C. difficile UK14, ATCC 43255, and ATCC 9689 strains. While another choice treatment of C. difficile infections, metronidazole inhibits the spore production in the ATCC 9689 strain and enhances it in the ATCC 53255 (27, 37). In this study, the VAN inhibits spore production in all of the clinical isolates (non-toxigenic and toxigenic) and ATCC 9689 strain. The rate of decline was about 1.5 - 3 Log/mL depending on the isolates and period of incubation. The non-toxigenic isolates and the toxigenic strains (clinical isolate and ATCC 9689) have similar behavior in the presence of VAN.
The effect of CLI and CAZ on spore production has not yet been explored. However, tigecycline as a protein inhibitor has reduced sporulation in the hypervirulent C. difficile ribotype 027. While it has enhanced the number of spores in ATCC 9689 strain (27). The spore production of three clinical isolates in the sub-MIC of CLI and CAZ has a similar pattern, and even more similar to VAN. The data show the CLI and CAZ inhibit the spore production in non-toxigenic isolates in the same manner of toxigenic strains. The reason for either inhibition or reduction of the spore production in sub-MIC of antibiotic is not well understood. The previous studies impact on the expression of those genes related to sporulation (27, 37). Incidentally, the prevalence of the non-toxigenic strain of C. difficile is high in the clinical samples (5, 7-12, 14). It is a good property of the non-toxigenic strains, which still maintain the capability of sporulation. In particular, non-pathogenic strains are not only harmful but also they are considered useful.
5.1. Conclusions
In summary, the previous studies have insisted on sporulation in the hypervirulent strains. In this study, non-toxigenic strains were evaluated and the data revealed non-toxigenic isolates have the similar potential capability of sporulation in the absence or presence of the sub-lethal concentration of antibiotics. It was also postulated why the prevalence of non-toxigenic isolates in diarrheal samples is high. Although these strains have lost the potential of toxin production; they have kept the ability to produce spore.