1. Background
Candida vaginitis or vulvovaginal candidiasis (VVC) is the most common fungal infection of the lower female genital tract mucosa at the reproductive age of 20 - 40 years (1, 2). The disease has a worldwide distribution with higher prevalence among debilitated women (3-5). VVC is caused by different species of Candida that are normally found on the mucosal surface of a healthy vagina. Infection might be accompanied by several symptoms, including itching, burning, smelly, abnormal vaginal discharge (a texture like cottage cheese), and redness of the vulva. Hormonal fluctuations in pregnancy, the use of widespread antibiotic therapy, oral contraceptives, obesity, and diabetes mellitus are common predisposing factors for the infection (5-9). Up to 70% of women have experienced VVC at least once during their lives. Additionally, recurrent vulvovaginal candidiasis (RVVC) occurs when ≥ 4 episodes of infection happen in a one - year period (5, 10, 11). Researchers believe that 5 - 8% of adult women have RVVC (5, 6, 11). Several studies have shown that the frequency of VVC varies in different provinces of Iran, from 4.8 to 73% (12-18).
The definitive diagnosis of VVC based on signs and symptoms is difficult due to similarities with other vaginal diseases, such as trichomoniasis and bacterial vaginosis. Therefore, mycological examinations of vaginal discharge are crucial for the diagnosis of infection and identification of causative agents (19). Recent reports represented an increasing trend in the rate of non-Candida albicans species isolated from VVC such as C. glabrata, C. tropicalis, and C. krusei (9, 20, 21). As mentioned by previous studies, inherent and acquired resistance by some non-C. albicans species are the two important reasons for the increased incidence of these species (22, 23). Usually, a single Candida sp. is isolated from a vaginal specimen; nonetheless, there are reports of more than one species isolated from the same patients with vulvovaginal candidiasis (2% - 5%) (24).
It is known that some Candida sp. have intrinsic resistance to antifungal agents and other species acquire resistance during VVC therapy period (25-27). These problems in the treatment of VVC, in addition to exerting physical and psychological adverse effects on the individual, can increase the possibility of infertility, pelvic inflammatory disease, and pelvic abscess. Moreover, inattention to this disease during pregnancy may lead to abortion, premature infant birth, postpartum infections, and systemic inflammation (7, 14).
2. Objectives
Due to the lack of sufficient information regarding the prevalence of different kinds of VVC and the type of causative species in Ahvaz, southwest of Iran, the aim of the present study was to determine the prevalence of VVC and the frequency of Candida sp. in women with signs and symptoms of vaginitis referring to the midwifery clinics in Ahvaz during 15 months from January 2017 to March 2018.
3. Methods
3.1. Study Area and Design
Ahvaz is the seventh most populous city in Iran with a population of 1302591 based on the 2017 census. It has an area of 518 km2, and has eight municipality districts. A descriptive cross-sectional study was conducted among women with symptoms of vaginal candidiasis attending the midwifery clinics in all municipality districts of Ahvaz between January 2017 and March 2018. A questionnaire was used to gather data such as age, predisposing diseases, oral contraceptive consumption, prior antifungal therapy, clinical signs and symptoms, and physiologic conditions (pregnant or non-pregnant) for each patient. In this study, VVC was determined based on a combination of women’s clinical signs and symptoms with laboratory culture and isolation of Candida sp. and its species definition. Besides, RVVC was considered as ≥ 4 episodes of symptomatic VVC per year.
3.2. Sample Collection and Identification
The identification of different species of Candida was based on the guidelines of the Center for Disease Control and Prevention (CDC) (28). The CDC criteria for laboratory diagnosis of VVC include the presence of pseudohyphae, true hyphae, yeasts cells or budding cells in the wet mount slide (10% KOH preparation), and a positive culture from each vaginal discharge. Vaginal specimens were collected with sterile cotton swabs from the endocervix. The first vaginal swab was used for direct examination and the second one was directly inoculated on CHROMagar Candida TM (CHROMagar, Paris, France) and transferred to the Department of Medical Mycology, affiliated to Ahvaz Jundishapur University of Medical Sciences. The culture media were incubated at 37°C for 48 - 72 hours.
3.3. Classical Identification
All Candida isolates were initially screened by phenotypic traits including their color on CHROMagar Candida, the formation of chlamydoconidia and true hyphae on corn meal agar (Difco, USA) with 1% Tween 80, germ tube formation in human serum, and growth at 45°C. In addition, all strains were subcultured on Sabouraud dextrose agar slants (BioLife, Italia) and preserved at the room temperature for further analysis.
3.4. Molecular Identification
The DNA of each yeast isolate was extracted using the simple boiling method and subjected to PCR-RFLP (21, 29). The fungal universal ITS1/ITS4 primer pair (30) was used to amplify the whole sequences spanning the conserved 5.8S rDNA region and flanking spacers (ITS1 and ITS2). The reaction mixtures contained 1 μL of each primer, 12.5 μL premix (Amplicon, Denmark), 3 μL of template DNA, and 7.5 μL distilled water. The amplification program included an initial denaturation cycle of 95°C for 5 minutes, 35 cycles of 95°C for 35 seconds, 58°C for 30 seconds, and 72°C for 1 minute, and a final extension of 72°C for 10 minutes. Subsequently, the products were subjected to digestion with MspI restriction enzyme (Thermo Fisher Scientific, Waltham, MA, USA). The final reaction included 6.5 μL of PCR product, 0.5 U of restriction enzyme MspI, 1 μL of digest buffer (Thermo Fisher Scientific, USA), and 7 μL distilled water. Then, incubation was done at 37°C for 16 h and digested products were electrophoresed on a 1.8% agarose gel. The identification of each isolate was accomplished by comparing the obtained banding patterns with those profiles demonstrated in the previous report for Candida sp. (29).
3.5. Statistical Analysis
The chi-squared test was applied using the SPSS software (version 22.0) to determine the effect of low-dose estrogen use on the occurrence of VVC. A P value of < 0.05 was considered as the significance level.
4. Results
In the present study, 493 patients in the age range of 15 to 64 years were examined for VVC. Among the patients, 473 (95.9%) were non-pregnant and the rest was pregnant. From among 493 suspected cases, 196 (39.76%) showed VVC by culture examinations. Moreover, nine (4.6%) cases were RVVC with at least four recurrent infections during one year. Totally, 55% (11 of 20) and 39.1% (185 of 473) of pregnant and non-pregnant women were positive for Candida vaginitis, respectively. In addition, the prevalence of VVC in eight different municipality districts of Ahvaz city ranged between 27.3 and 42.8%, with a mean of 36.4%. This survey showed that VVC occurred most frequently in the age group of 21 - 30, followed by the age group of 31 - 40 whereas the lowest frequency of VVC was seen in > 50-year-old individuals (Table 1).
| Age Group | Examined Patients, No. (%) | VVC Patients, No. (%) |
|---|---|---|
| ≤ 20 | 53 (10.8) | 22 (11.2) |
| 21 - 30 | 235 (47.7) | 104 (53.1) |
| 31 - 40 | 146 (29.6) | 47 (24) |
| 41 - 50 | 49 (9.9) | 19 (9.7) |
| > 50 | 10 (2) | 4 (2) |
| Total | 493 (100) | 196 (100) |
Abbreviation: VVC, vulvovaginal candidiasis.
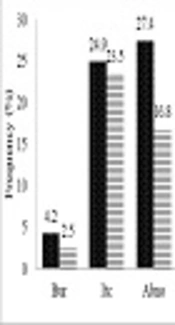
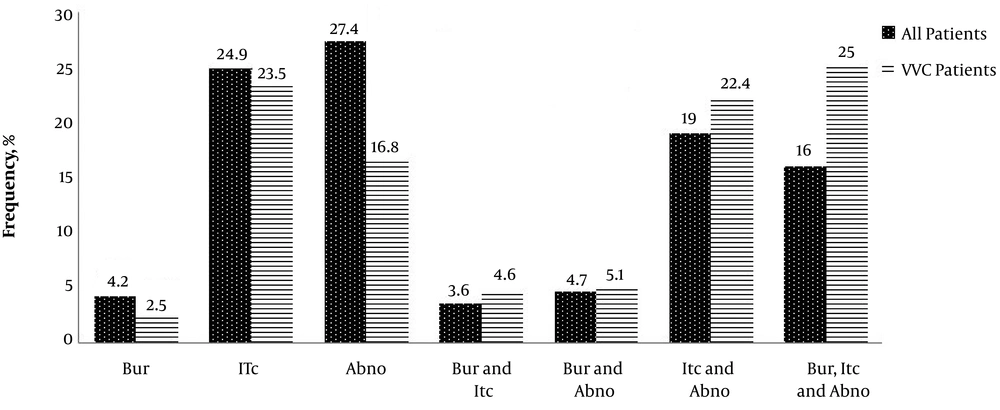
Six out of 11 (54.5%) pregnant patients with VVC were in the first trimester of pregnancy (the first 13 weeks) and 27.3% (three out of 11) in the second trimester (weeks 13 to 28). Only were 18.2% (two out of 11) of the VVC patients in the third trimester (weeks 29 to 40) of pregnancy whereas all (100%) of the pregnant women without VVC were in the third trimester of pregnancy. Our study showed that 84 (42.9%) out of 187 low-dose estrogen users (LD) had VVC and there was no statistically significant relationship between the use of LD and the occurrence of VVC (P = 0.067). Itching (75%) was the most prevalent sign and symptom in the VVC patients, followed by abnormal discharges (51%), burning (32.24%), and burning concomitant with itching and abnormal discharge (25%) (Figure 1).
In this investigation, 211 Candida strains were isolated from 196 patients with VVC. Moreover, multispecies were found in 14 (7.1%) patients with VVC. The most common isolated species was C. albicans with 150 cases (71.1%), followed by C. glabrata with 43 (20.4%), C. krusei with nine (4.3%), C. kefyr with five (2.4%), C. lusitaniae with three (1.4%), and C. tropicalis with one case (0.5%). Candida albicans was the causative agent in 77.8% (seven cases) of the patients with RVVC, C. glabrata in 11.1% (one case), and multispecies of C. albicans, C. glabrata, and C. krusei in one case. As shown in Table 2, C. albicans was the most commonly isolated species from both pregnant and non-pregnant patients, followed by C. glabrata.
| Candida Species | Pregnant Patients, No. (%) | Non-Pregnant Patients, No. (%) |
|---|---|---|
| Candida albicans | 8 (72.7) | 129 (70.2) |
| C. glabrata | 2 (18.2) | 28 (15.1) |
| C. krusei | - | 7 (3.7) |
| C. kefyr | - | 4 (2.2) |
| C. lusitaniae | - | 3 (1.1) |
| C. tropicalis | - | 1 (0.5) |
| C. albicans + C. glabrata | - | 11 (6.1) |
| C. albicans + C. kefyr | - | 1 (0.5) |
| C. glabrata + C. krusei | 1 (9.1) | - |
| C. albicans + C. glabrata + C. krusei | - | 1 (0.5) |
| Total | 11 (100) | 185 (100) |
5. Discussion
Vulvovaginal candidiasis is a common vaginal yeast infection affecting reproductive-aged women. Although the disease has a worldwide distribution, some socioeconomic factors can affect its frequency. Therefore, updated epidemiological data from each area are the most important tools for health service planning managers. In the present study, a different frequency of VVC (39.76%) was reported when compared to our previous studies in Ahvaz in 2016 (28.3%) and 2010 (49%) (15, 21). The frequency of VVC in other cities in Iran was reported as 62.1%, 43.3%, 40%, 26%, and 4.8% from Ilam, Babol, Arak, Hamadan, and Zanjan, respectively (12, 13, 16, 31, 32).
We found that the frequency of VVC in our study is higher than the rates found in Nigerian (14.0%), Indian (20.47%), and Turkish (16%) studies (6, 33, 34). On the other hand, a very high prevalence of VVC (84.5%) was reported by Ugwa from North-West Nigeria (35). In contrast, the lowest frequency of infection was recorded in Brazil (5.44%) (36). Our study found the different frequency of VVC (from 27.3% to 42.8%) in the eight municipality districts of Ahvaz. This variation could be due to a different number of patients from each district, socioeconomic status of sample patients, patients’ knowledge about personal hygiene, and self-treatment.
In our study, the highest frequency of VVC was seen in patients with ≤ 40-years-old while patients aged ≥ 41 years had the lowest frequency. Likewise, the most frequency of VVC was found in the age group of 21 - 30 years, which is similar to the findings of Hedayati et al. from Iran and Adesiji et al. from Nigeria (14, 37). Increased sexual activity in these age groups may be a reason for higher VVC (8). The prevalence of VVC among pregnant patients in our study was 55% that is similar to the study by Nwosu et al. (56.3%) from North-Eastern Nigeria (38). Nevertheless, other studies reported a different prevalence of VVC among pregnant patients, 70.2% from Saudi Arabia (7), 60.76% from North-West Nigeria (39), and 31.6% from Pakistan (40). These differences may be because of long-term treatment with antibiotics and different knowledge levels about personal health in different areas.
In our study, although the frequency of VVC was found higher among pregnant women (55%) than in non-pregnant women (39.1%), there was no statistically significant difference between them (P = 0.155). Higher estrogen and progesterone levels during pregnancy can explain the cause of the increase of developing VVC (41). In addition, our results demonstrated that the prevalence of VVC was higher in the first trimester (54%) and second trimester (27%) of pregnancy than in the third trimester (18%) while Kanagal et al. found that the second trimester had the highest prevalence of VVC (54%), followed by 30% in the third trimester and 16% in the first trimester (42). Similarly, Holzer et al. reported the more occurrence of VVC in the first trimester (63%) than in the second trimester (37%) (43).
Our study indicated that the frequency of VVC among LD users was 42.9%. Cheraghi et al. reported that the highest rate of VVC (34.3%) was seen in LD use among other contraceptive methods (44). Similar to previous studies (14-16, 45, 46), C. albicans was the most prevalent species (71.1%), followed by non-C. albicans species (28.9%); among the latter group, C. glabrata (20.4%) was the most common species. This finding is similar to other studies (14, 15, 46). Contrary to our study, some recent studies have shown that VVC due to non-C. albicans species is in the rise (14, 16, 22). The increased frequency of non-C. albicans species is probably related to self-curing, unnecessary consumption of antifungal agents, and increased resistance of some non-C. albicans species to fluconazole (22).
5.1. Conclusion
It was found that VVC is relatively a common gynecologic problem in Ahvaz. Although the incidence of non-C. albicans species in VVC has increased, C. albicans is still the predominant species isolated from VVC.