1. Background
Oral candidiasis, as one of the most common opportunistic fungal infections, can appear in individuals with immunodeficiency, diabetes, and other predisposing conditions (1). Twelve of 14 species of non-albicans Candida are recognized as the causes of candidiasis but Candida albicans is the most common one (2). The oral cavity, gastrointestinal tract, and other mucous membranes are the hosts of C. albicans, with other yeasts growing as the normal flora, leading to hematogenous seeding with yeast cells when the immune system is suppressed. According to epidemiological studies, non-albicans Candida species including C. tropicalis, C. parapsilosis, C. krusei, and C. glabrata have repeatedly emerged as human pathogens (3, 4).
Some Candida spp. are the normal flora of the oral cavity in healthy people. Strain C. albicans can be switching between the yeast and the pseudo-hyphal forms enabling it to cause diseases in immunosuppressed people ranging from superficial infection to deep disseminated infection (5, 6). Candida albicans has multiple virulence factors including adhesion, invasion, yeast-hyphal transition, biofilm formation, phenotypic switching, and secretion of hydrolytic exoenzymes contributing to its colonization and pathogenicity (7). Candida albicans is overgrown by exoenzymes that accelerate the adhesion, penetration, and invasion of host tissue (8, 9). In vivo study, the filamentous form of C. albicans was used to upregulate secreted aspartyl proteinase (SAP). In vitro study, C. albicans secreted SAP while culturing on media containing bovine serum albumin protein as the nitrogen source.
Among other extracellular hydrolytic enzymes are phospholipase enzymes that damage phospholipids in the membrane of epithelial cells resulting in cell damage, lysis, and invasion (10). There are four types of phospholipase enzymes (PLA, PLB, PLC, and PLD) (11). The hemolysin enzyme is secreted by Candida species and destroys blood cells and releases iron; thus, the increase of iron and its transfer to Candida yeast can cause the growth of the yeast and enhance fungal infection (12). In 2008, Almeida et al. showed that C. albicans caused more damage to oral epithelial cells containing high levels of ferritin than to cells with low iron levels (13). In vitro, esterase activity is considered as one of the pathogenicity factors of the yeast and can be induced in media containing tween 80.
Phospholipase enzymes are related to cell damage, adhesion, penetration and so, invasion (10). They act by destroying phospholipids in epithelial cells resulting in cell membrane damage and lysis (11). Elemental iron stored in the cell during cell destruction is acquired by Candida through the production of hemolysin enzymes and metabolism, growth, and enhancement of infections occur after iron chelation and transport to the fungus (12). In vivo study, hemolysis is the capability of C. albicans to use hemoglobin in erythrocytes as a source of iron (14). In 2008, Almeida showed that, compared to cells containing low iron levels, C. albicans brought greater damage to oral epithelial cells containing an elevated concentration of ferritin (13). By acting on the ester bonds in glycerides, esterase enzymes can hydrolyze triacylglycerol (12). In vitro, esterase activity is considered one of the pathogenicity factors of this yeast and can be induced in media containing tween 80 (14).
2. Objectives
The aim of the study was to investigate the activity of enzymes including esterase, phospholipase, proteinase, and hemolysin secreted by Candida strains isolated from the oral cavity of cancer patients with oral candidiasis and normal people.
3. Methods
In this study, 72 Candida isolates were tested among which, 36 isolates were related to oral candidiasis patients under chemotherapy in a previous study including C. albicans (n = 26; 72.2%), C. glabrata (n = 5; 13.8%), C. kefyr (n = 3; 8.3%), C. krusei (n = 1; 2.8%), and C. stellatoidea (n = 1; 2.8%) (15). Moreover, 36 isolates of Candida sp. were obtained from normal oral flora of healthy people by two sterile swabs. To compare the two groups, the participants were controlled for age and sex.
3.1. Preparation of Suspension of Candida Isolated from Healthy People
All specimens were cultured on Sabouraud dextrose agar (SDA, Merck, Germany) and incubated aerobically at 37°C for 24 - 48 hours. The CHROM agar Candida medium (CHROMagar HiMedia, Mumbai, India) was used to identify Candida isolates. PCR-RFLP using an Msp1 restriction enzyme on the internal transcribed spacer 1 - 5.8 ribosomal DNA-ITS2 (ITS1-5.8S rDNA-ITS2) rDNA region was used to identify isolates to the species level (16). All the isolates of Candida species were subcultured at 30°C for 24 hours in SDA plates; then, a suspension was prepared adjusted at 0.5 McFarland standard (1 × 106 to 5 × 106 cells per mL) at the 530 nm wavelength.
3.2. Detection of Some Virulence Factors
3.2.1. Determination of Phospholipase Activity
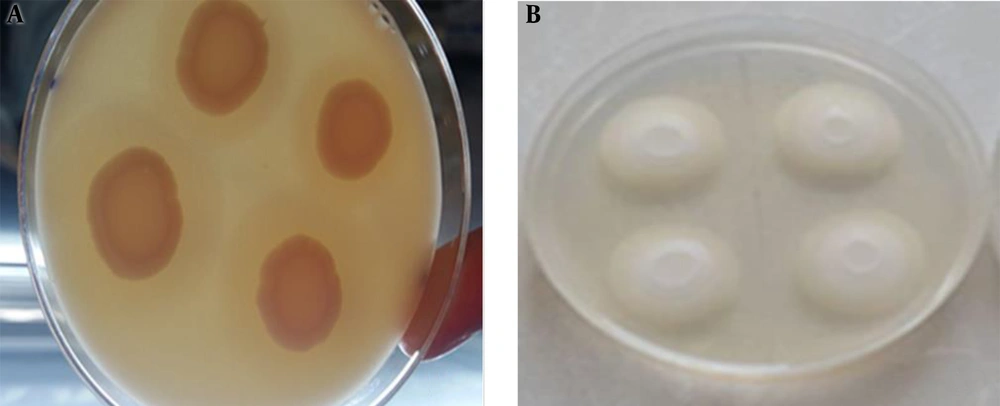
The test medium contained 65 g SDA, 58.4 g NaCl, and 5.5 g CaCl2. All were dissolved in 980 mL of distilled water and the solution was sterilized by autoclaving at 121°C psi for 12 minutes. Egg yolk centrifugation was conducted at 5000 g for 30 minutes. The supernatant was added to the prepared medium at the rate of 2%, mixed, and dispensed in plates. An aliquot (10 µL) of the yeasts suspension was inoculated on the test medium and incubated at 37°C for four days. Next, due to the removal of lipids by Candida sp., the phospholipase activity was determined as a clear zone around each colony of Candida sp. (17, 18).
3.2.2. Determination of Proteinase Activity
The test medium was composed of 60 mL of a solution containing 0.04 g MgSO4.7H2O, 0.5 g K2HPO4, 1 g NaCl, 0.2 g yeast extract, 4 g glucose, and 0.5 g BSA (bovine serum albumin) and the pH was adjusted to 4.0. Filtration was used to sterilize the solution, followed by mixing with 140 mL of molten agar. An aliquot (10 µL) of the yeasts suspension was inoculated on the test medium and incubated at 37°C for seven days. The proteinase activity was measured as the diameter of clear zones around the colonies (19, 20). Proteinase activity was determined using the amido black staining solution.
3.2.3. Determination of Esterase Activity
The test medium containing 10 g peptone, 5 g NaCl, 0.1 g CaCl2, and 15 g agar was prepared by dissolving in 1000 mL distilled water, with pH adjusted to 6.5. It was cooled down to about 50°C after being sterilized by autoclaving at 121°C psi for 15 minutes, followed by adding 5 mL of sterilized Tween 80. An aliquot (10 µL) of the yeasts suspension was incubated at 37°C for five days after being inoculated on the test medium. The precipitation zone around the colonies was used to determine the lipase activity (21, 22).
3.2.4. Determination of Hemolysin Activity
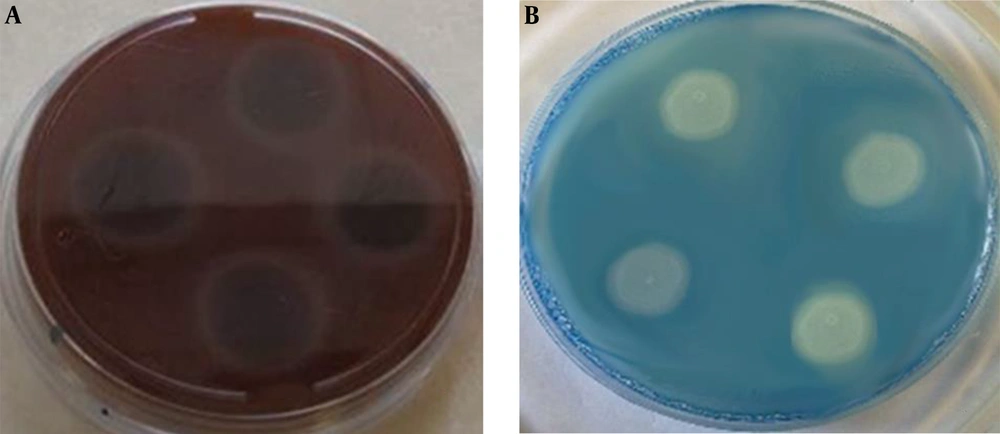
Human blood (7%) and glucose (3%) with a final pH of 5.6 were added and dispensed into sterile Petri dishes when the medium was cooled down to 50 - 55°C. An aliquot (10 µL) of the yeasts suspension was inoculated on the test medium and incubated at 37°C for 48 hours. This medium was used to detect the ability of isolates to produce hemolysin (22).
3.3. Enzymatic Assay
In this study, the relevant protocols were used to determine the in vitro proteinase, phospholipase, esterase, and hemolysin activities of the 72 isolates (21, 23, 24). The ratio of the colony diameter to the diameter of the unstained zone was used to measure the proteinase and hemolysin activities in mm (23, 25, 26). The phospholipase and esterase activities were defined as the ratio of the colony diameter to the precipitation zone diameter. The measurement of enzymatic activity was based on the enzymatic activity index (EAI) as stated above (27, 28). The ranges of activity were established according to the EAI index: positive ≤ 0.99 and negative EAI = 1.
3.4. Statistical Analysis
SPSS version 21 software was used for statistical analysis. The EAI levels were compared between Candida species by using one-way analysis of variance (ANOVA) and post hoc test (LSD). P values of < 0.05 were considered statistically significant.
4. Results
Candida albicans was the most frequently isolated species in 31 (86%) and 26 (72%) specimens of oral cavities of healthy people and cancer patients, respectively. The other detected Candida sp. were C. glabrata 3 (8%), and C. krusei and C. tropicalis 1 (3%).
4.1. Phospholipase Activity
Of the 72 studied isolates, 38 (53%) C. albicans isolates had phospholipase activity including 23 (64%) from control subjects and 15 (42%) from patients. The enzyme did not have activity in three C. glabrata isolates from control subjects. Of the three isolates of C. glabrata and C. kefyr isolated from patients, two showed phospholipase enzyme activity (Figure 1A). In the patient group, four isolates had phospholipase enzyme activity. Among Candida kefyr isolates from patients, two had phospholipase enzyme activity. The phospholipase enzyme activity was positive in four (80%) C. glabrata and two (67%) C. kefyr isolates from the patient group (Figure 1A). The mean EAI for phospholipase was 0.7750 and 0.7950 in the cancer patient and normal groups, respectively (Table 1). According to the results, the average activity of the phospholipase enzyme was more in patients than in control subjects, but did not show a statistically significant difference between the two groups (P > 0.05).
| Candida Species | Normal Individuals | Cancer Patients | Total | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Candida albicans | 8 (22) | 23 (64) | 11 (31) | 15 (42) | 19 (26) | 38 (53) |
| C. glabrata | 3 (8) | 0 | 1 (3) | 4 (11) | 4 (6) | 4 (6) |
| C. kefyr | 0 | 0 | 1 (3) | 2 (6) | 1 (1) | 2 (3) |
| C. krusei | 1 (3) | 0 | 0 | 1 (3) | 1 (1) | 1 (1) |
| C. tropicalis | 0 | 1 (3) | 0 | 0 | 0 | 1 (1) |
| C. stellatoidea | 0 | 0 | 1 (3) | 0 | 1 (1) | 0 |
| Total | 12 (33) | 24 (67) | 14 (39) | 22 (61) | 26 (36 ) | 46 (64) |
| 36 (100) | 36 (100) | 72 (100) | ||||
| Mean EAI | 0.7950 | 0.7750 | ||||
Abbreviation: EAI, enzymatic activity index.
aValues are expressed as No. (%).
bP value = 0.48.
4.2. Esterase Activity
The enzyme activity was positive in 31 (86%) C. albicans isolates from healthy people and 22 (61%) from patients. Three (8%) C. glabrata isolates from control subjects did not show the enzymatic activity. Out of five isolates from patients, three (8%) showed esterase activity. Of three (8%) isolates of C. kefyr from patients, 100% showed esterase activity (Figure 1B). The mean EAI for esterase was 0.7186 and 0.6142 in the cancer patient and normal groups, respectively (Table 2). According to the results, the production of esterase enzyme in Candida species in the study population could not be the cause of oral candidiasis.
| Candida Species | Normal Individuals | Cancer Patients | Total | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Candida albicans | 0 | 31 (86) | 4 (11) | 22 (61) | 4 (6) | 53 (74) |
| C. glabrata | 3 (8) | 0 | 2 (6) | 3 (8) | 5 (7) | 3 (4) |
| C. kefyr | 0 | 0 | 0 | 3 (8) | 0 | 3 (4) |
| C. krusei | 0 | 1 (3) | 0 | 1 (3) | 0 | 2 (3) |
| C. tropicalis | 0 | 1 (3) | 0 | 0 | 0 | 1 (1) |
| C. stellatoidea | 0 | 0 | 0 | 1 (3) | 0 | 1 (1) |
| Total | 3 (8) | 33 (92) | 6 (17) | 30 (83) | 9 (13) | 63 (87) |
| 36 (100) | 36 (100) | 72 (100) | ||||
| Mean EAI | 0.6142 | 0.7186 | ||||
Abbreviation: EAI, enzymatic activity index.
aValues are expressed as No. (%).
bP value = 0.009.
4.3. Hemolysin Activity
The hemolysin enzyme activity was positive in 30 (83%) and 26 (72%) C. albicans isolates from the control and patient groups, respectively. Of eight (100%) isolates of C. glabrata in both control and case groups, the hemolysin enzyme activity was positive in three (8%) and five (14%) isolates, respectively, showing 100% activity in the two groups. Three (8%) C. kefyr isolates from patients showed 100% hemolysin activity (Figure 2A). The mean EAI for hemolysin factor was 0.5756 and 0.6317 in cancer patients and normal groups, respectively (Table 3). According to the results, the hemolysin activity of Candida species was significantly higher in the patient group than in the control group (P < 0.05).
| Candida Species | Normal Individuals | Cancer Patients | Total | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Candida albicans | 1 (3) | 30 (83) | 0 | 26 (72) | 1 (1) | 56 (78) |
| C. glabrata | 0 | 3 (8) | 0 | 5 (14) | 0 | 8 (11) |
| C. kefyr | 0 | 0 | 0 | 3 (8) | 0 | 3 (4) |
| C. krusei | 0 | 1 (3) | 0 | 1 (3) | 0 | 2 (3) |
| C. tropicalis | 0 | 1 (3) | 0 | 0 | 0 | 1 (1) |
| C. stellatoidea | 0 | 0 | 0 | 1 (3) | 1 (1) | |
| Total | 1 (3) | 35 (97) | 0 | 36 (100) | 1 (1) | 71 (99) |
| 36 (100) | 36 (100) | 72 (100) | ||||
| Mean EAI | 0.6317 | 0.5756 | ||||
Abbreviation: EAI, enzymatic activity index.
aValues are expressed as No. (%).
bP value = 0.004.
4.4. Proteinase Activity
Of the 43 C. albicans isolates positive for proteinase activity, 26 were related to control subjects and 15 to patients. Candida glabrata isolated from the controls showed 100% activity of the proteinase enzyme while three out of five isolates from the patients showed the activity of this enzyme. Of the three C. kefyr isolates from the patients, two isolates showed the proteinase activity (Figure 2B). The mean EAI for proteinase was 0.7558 and 0.7531 in cancer patients and normal group, respectively (Table 4). According to the results, the mean activity of the proteinase enzyme was not significantly different between the two groups (P > 0.05).
| Candida Species | Normal Individuals | Cancer Patients | Total | |||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | |
| Candida albicans | 5 (14) | 26 (72) | 9 (25) | 17 (47) | 14 (19) | 43 (60) |
| C. glabrata | 0 | 3 (8) | 2 (6) | 3 (8) | 2 (3) | 6 (8) |
| C. kefyr | 0 | 0 | 1 (3) | 2 (6) | 1 (1) | 2 (3) |
| C. krusei | 1 (3) | 0 | 0 | 1 (3) | 1 (1) | 1 (1) |
| C. tropicalis | 1 (3) | 0 | 0 | 0 | 1 (1) | 0 |
| C. stellatoidea | 0 | 0 | 0 | 1 (3) | 0 | 1 (1) |
| Total | 7 (19) | 29 (81) | 12 (33) | 24 (67) | 19 (26) | 53 (74) |
| 36 (100) | 36 (100) | 72 (100) | ||||
| Mean EAI | 0.7531 | 0.7558 | ||||
Abbreviation: EAI, enzymatic activity index.
aValues are expressed as No. (%).
bP value = 0.14.
5. Discussion
Infections caused by Candida species are increasing in immunocompromised patients. Oral candidiasis is one of the most common opportunistic infections in this group of patients (29-31). The main cause of candidiasis is C. albicans although non-albicans such as C. glabrata and C. krusei are increasing (32). In the present study, among 36 isolates from healthy subjects 31 (86%) C. albicans and five (14%) non-albicans isolates were identified using phenotypic and molecular methods. Therefore, in the control group, similar to patients undergoing chemotherapy, C. albicans showed the most identified isolates. C. albicans is known as an opportunistic pathogen causing the disease development by attacking the host tissue (8). Candida albicans can exist as normal flora in the mouth of all individuals under normal conditions but this yeast can cause disease in the oral cavity in certain conditions, especially when the body resistance is reduced (33).
One of the virulence factors in oral candidiasis is the adhesion of the yeast to the host cell surface. In this regard, various hydrolytic enzymes have been identified in Candida sp. such as phospholipase, proteinase, and esterase involved in Candida colonization in the host cell (34). In this study, Candida isolates from patients with oral candidiasis showed variable enzymatic activities compared to Candida isolates from the control group. Thus, almost 100% of Candida species possessed phospholipase and proteinase activity and the secretion of these enzymes was higher in the oral candidiasis patients under chemotherapy than in the oral cavity normal flora of healthy subjects although with no statistically significant difference. In a study by Tsang et al. investigating oral candidiasis isolates from diabetic patients compared to a control group, they found that the secretion of these enzymes was positive in both groups with more secretion in the patient group (21).
In a study by Price et al. examining how these enzymes can act on the host, it was found that by digesting the host cell membrane, these enzymes can cause sustained adhesion of the yeast cell to the host and ultimately lead to infection. They showed that the development of the disease by different strains of Candida is dependent on the activity level of the enzyme (24, 35). In our study, the mean hemolytic activity of Candida species was significantly higher in chemotherapy patients than in the control group, which was consistent with the studies conducted by Manns et al. (23) and Watanabe et al. (36). They reported that hemolysin enzymes in different strains of Candida could cause iron release from red blood cells and the free iron in saliva is necessary for the pathogenicity of various strains of candida (13, 23, 36).
In the present study, all Candida species in both groups had esterase activity and the mean activity of this enzyme in healthy subjects was significantly higher than that of the patient group. Kumar et al. in a study detected different levels of esterase activity in different Candida sp. They found the highest activity of this enzyme in C. albicans species (37). Therefore, in the present study, the higher mean total activity of esterase in healthy individuals could be due to the more prevalence of C. albicans isolates than their prevalence in the patient group. Recently, Noori et al. in a study on Candida species isolated from individuals with vulvovaginal candidiasis showed no significant relationship between esterase production and the presence of VVC. Their results are similar to our study findings (38). Most Candida species isolated from humans secrete three groups of hydrolytic enzymes including phospholipase, proteinase, and esterase following the mycelial growth, which can facilitate their active penetration into host cells; they can be identified by quantitative and qualitative methods as virulence factors (39, 40).
In this study, C. albicans isolates were more prevalent than other Candida species in both control and case groups and C. albicans species showed the highest secretion of these four enzymes in both groups. A study by Issa et al. found that C. albicans species isolated from the oral cavity and other parts of the human body have the potential for more enzymatic secretion than other Candida species (41). However, different studies have reported that Candida strains isolated from infected areas in patients and healthy people show a significant potential for the secretion of hydrolytic enzymes. Therefore, it can be concluded that all commensal strains are opportunistic and are able to develop the disease in favorable conditions (8, 42).
5.1. Conclusions
The activity of the extracellular C. albicans enzymes in both groups was more than the activity of non-albicans, explaining the role of these factors in the development of diseases caused by these yeasts, especially C. albicans. Phospholipase, proteinase, and hemolysin activity of Candida species was higher in patients than in healthy people and hemolytic activity was also significantly higher in patients than in healthy subjects (P < 0.05). Esterase activity of Candida species was significantly higher in patients than in healthy subjects. How the pathogenesis of Candida sp. is working is essential to develop new antifungal agents and determine the cause of drug resistance and management of patients. This study helped us to better understand the mechanism of Candida sp. However, more research is needed in this regard.