1. Background
Staphylococcus aureus is one of the most frequent foodborne pathogens causing various infections such as wound infection, septicemia, meningitis, toxic shock syndrome (TSS), and osteomyelitis in humans (1, 2). Staphylococcus aureus produces different toxins and enzymes including enterotoxins, DNase, coagulase, lipase, TSST-1 (tst), and hemolysin involved in the pathogenesis of the diseases (3). The enterotoxins (sea to see and seg to sej) are heat-resistant (4, 5). These toxins can cause food poisoning, staphylococcal gastroenteritis, and diverse autoimmune and allergic diseases (6, 7). Staphylococcus aureus also secretes protein A (spa) and second immunoglobulin-binding protein (sbi), virulence factors associated with staphylococcal pneumonia, and hypodermic infection (8, 9). Therefore, the sea, spa, tst, and sbi genes encode important virulence factors that are regulated by accessory gene regulators (agr) and two-component signal transduction system (sae) in response to environmental stimulating factors (10-12).
Methicillin-resistant S. aureus (MRSA) like other multidrug-resistant bacteria is now resistant to many antimicrobial agents and cause serious infections in inpatients and outpatients (6, 13). These isolates from human sources and dairy products are related to foodborne diseases (e.g., diarrhea) in humans who consume contaminated dairy products. Therefore, there is an increasing need to study alternative agents with maximum efficacy and minimum side effects (14, 15).
Probiotic bacteria like Lactobacillus species (Lactobacillus reuteri, L. fermentum, and L. plantarum), as a beneficial group of microbiota, play a considerable role in human health (16, 17). Lactic acid bacteria (LAB) demonstrate the inhibitory and therapeutic effects on some intestinal pathogenic infections, antibiotic-associated gastrointestinal complications, diarrhea, allergies, cancers, and certain inflammatory and immunomodulatory disorders (18-20). The supernatant of the LAB culture includes aroma components, fatty acids, organic acids, low-molecular-mass compounds, and hydrogen peroxide that can kill pathogens (21). In recent studies, the effect of the supernatant of LAB has been probed on the inhibition of the growth of pathogenic bacteria such as S. aureus and less attention has been put on the effect of the supernatant on the expression of virulence factors.
2. Objectives
In this study, we evaluated the effect of the CFS of three probiotic LAB (L. reuteri, L. fermentum, and L. plantarum) on the gene expression regulator system (sae and agr A) and other genes (sea, tst, spa, and sbi) at the transcriptional level.
3. Methods
3.1. Bacterial Strains and Growth Conditions
The Methicillin-resistant S. aureus (MRSA) strain (ATCC 33591), L. reuteri (ATCC 23272), L. plantarum (ATCC 8014), and L. fermentum (ATCC 9338) were obtained from the Persian type culture collection (PTCC). Methicillin-resistant S. aureus was cultured in Muller Hinton Broth (MHB) at 37°C until it reached the exponential phase of growth. Lactobacilli were cultured in MRS (De Man, Rogosa, and Sharpe) broth at 37°C until they reached the stationary phase of growth. After a 24-h period, the lactobacilli cells were harvested by centrifuging at 10,000 × g for 10 min at 4°C. The pellet was discarded and the cell-free supernatants (CFS) were then adjusted to natural pH (pH = 7) with NaOH (1 N) and sterilized through a 0.22-µm filter. These supernatants were stored at -20°C for later use.
3.2. Microbial Interactions
The density and the number (CFU/mL) of MRSA in cultures were measured through the use of spectrophotometry at OD600. The 1/2 and 1/4 × CFS of Lactobacilli spp. were added to the medium (MHB) containing 107 CFU/mL of MRSA bacteria. After 6 and 12 h of incubation, the bacterial density was quantified at OD600, and then centrifuged at 12,000 × rpm/10 min at 4°C. The supernatants were removed and the pellet was used for RNA extraction. A lactobacilli-free culture of MRSA was used as control at the logarithmic phase of growth.
3.3. RNA Extraction and cDNA Synthesis
The RNA was extracted using a Rapid Bacterial RNA Isolation Kit (Bio Basic, Inc., Canada) according to the manufacturer’s instructions. The total RNA was solved in 30 µl of RNase-free water. To remove all DNA, the purified RNA was treated with DNase I, RNase-free (Cinna Gene, Iran) after extraction. The purity of RNA was determined by calculating A260/A230 and A260/A280 ratios (Denovix, NanoDrop, USA) and the integrity of total RNA was assessed with 1% agarose gel (data not shown). The cDNA was synthesized using a cDNA reverse transcription kit (Pars Tous, Iran).
3.4. Quantitative PCR
The quantitative polymerase chain reaction (qPCR) was performed using primers described previously (22-24). Briefly, all PCR assays were accomplished in a total volume of 20 µL, containing 10 µL of Sybr Green Mix (Takara Bio®, Japan), 0.1 µM of primers, 5 ng of cDNA, and deionized water. The RT-qPCR amplification was carried out by the Applied Biosystems 7500 and the Step One Plus Real-time PCR system (ABI, France). The cycling parameters for qPCR included an initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. We used LinRegPCR software to calculate the amplification efficiency of each sample (25). We entered the raw dataset of RT-qPCR outputs into LinRegPCR version 2017.1 software (https: //LinRegPCR.HFRC.nl). The performance and efficacy of all primers was evaluated separately on the qPCR data of samples. The housekeeping gene 16S rRNA was applied as an internal control. The expression ratio (fold) and the up-regulation and down-regulation of genes were calculated using the ΔΔCt formula. The data were represented as mean ± standard deviation (SD). All experiments were accomplished independently at least three times. Statistical analysis was performed using GraphPad Prism version 5.00 software (GraphPad software, Inc., CA, USA).
4. Results
While the growth of MRSA in the presence of CFS of lactobacilli was not inhibited over the six and 12-h periods of post-treatment, the level of gene expression was significantly reduced (Table 1) depending on the species of Lactobacillus, the supernatant concentration, and the incubation time.
| Species | Bacterial Growth at OD600 | ||
|---|---|---|---|
| 0 hb | 6 h | 12 h | |
| Staphylococcus aureus (MRSA) | 0.41 | 0.71 ± 0.021 | 0.986 ± 0.011 |
| +Lactobacillus reuteri (CFS) | 0.41 | 0.67 ± 0.031 | 0.88 ± 0.042 |
| +L. plantarum (CFS) | 0.41 | 0.656 ± 0.007 | 0.845 ± 0.012 |
| +L. fermentum (CFS) | 0.41 | 0.63 ± 0.01 | 0.82 ± 0.026 |
aValues are expressed as mean ± SD.
bStarting bacterial inoculum.
4.1. Gene Expression at 6-h Post-Treatment
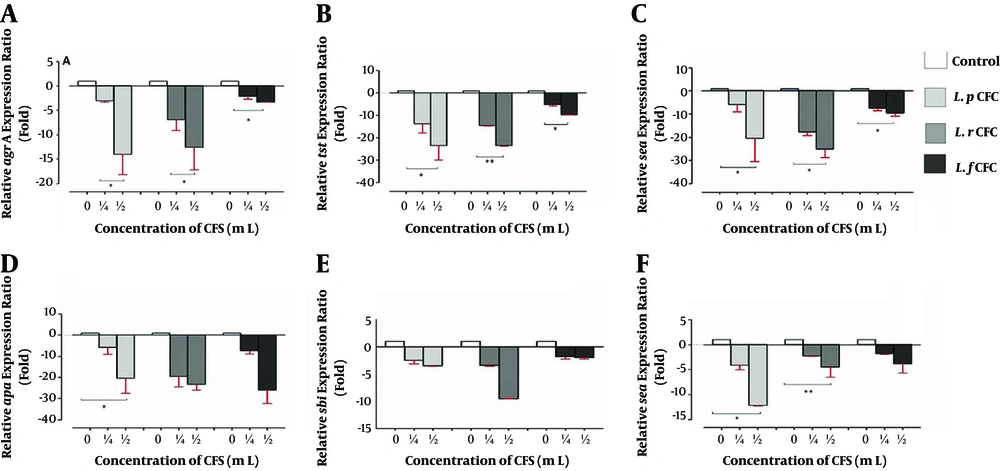
The attenuation of virulence gene expression of MRSA was observed at 6-h post-treatment (Figure 1). At this primary time-point, no significant change was observed in the expression of any of the study genes (Figure 1A, E, F, B, and C). By increasing the CFS concentration to 1/2 × CFS, the ratio of gene expression decreased significantly from 2.18 to 7.98 folds compared to that of the control in the spa, sea, sae, tst, and agr A genes in the presence of the CFS of all the three Lactobacilli studied (P < 0.05). The maximum reduction belonged to the sea gene (7.89 folds) (Figure 1C). However, by increasing the concentration of L. reuteri and L. fermentum CFS, there was no significant decrease in the expression of the sbi and spa genes (Figure 1E and D).
Relative gene expression ratios of MSRA in the presence of CFS of Lactobacillus spp. after 6-h incubation. A, agr A gene; B, tst gene; C, sea gene; D, spa gene; E, sbi gene; and F sae gene, at two different concentrations of three different CFS. Data are the average of three independent experiments performed in triplicate and reported as fold ± SD.
4.2. Gene Expression at 12-h Post-Treatment
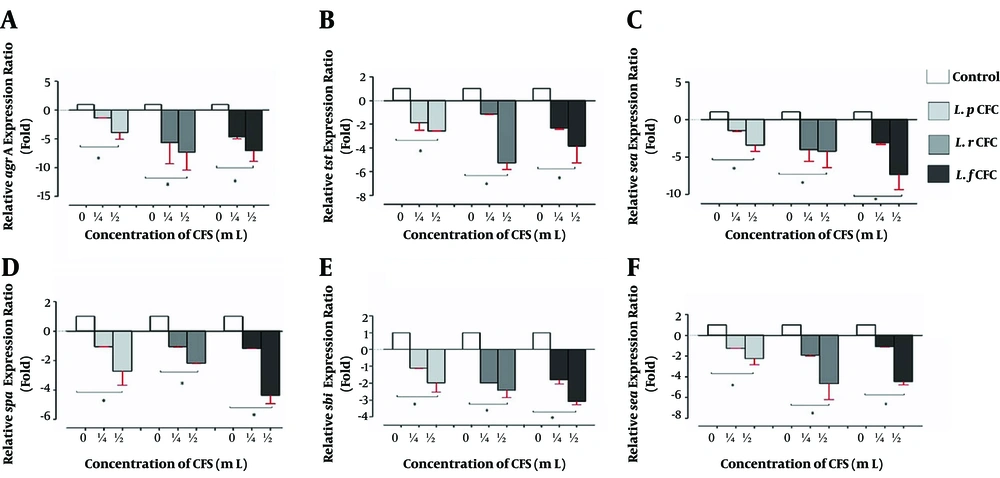
The expression levels of the studied genes also decreased at 12-h post-treatment. However, they were different from the expression levels at 6-h post-treatment (Figure 2). The expression ratios of the agr A, tst and sea genes in all types of CFS markedly decreased 12-h post-treatment in comparison with the control group at a concentration of 1/2 × CFS from 14.3 to 26.6 folds (Figure 2A-C). The significant reductions in the expression of the sae gene were also observed in the presence of L. plantarum and L. reuteri CFS (Figure 2F). However, by increasing the incubation time, L. fermentum CFS (1/2 and 1/4) could not significantly affect the expression ratios of the sae, sbi, and spa genes (Figure 2F and E). The maximum reduction, up to 23.5 folds, was observed in the tst and sea genes (Figure 2B and C).
Gene expression of MSRA in the presence of CFS of Lactobacillus spp. after 12-h incubation. A, agr A relative expression ratio; B, tst relative expression ratio; C, sea relative expression ratio; D, spa relative expression ratio; E, sbi relative expression ratio; and F, sae relative expression ratio at two different concentrations of three different CFS after 12 hours. Data are the average of three independent experiments performed in triplicate and reported as fold ± SD.
5. Discussion
Despite various studies conducted on the virulence genes of S. aureus aiming to reduce its adverse effects, this human pathogen is still considered an extremely prevalent pathogen worldwide (26, 27). The objective of this study was to compare the potential effects of CFS from three different probiotic lactobacilli including L. reuteri, L. fermentum, and L. plantarum on the expression of some virulence factor encoding genes of MRSA at different incubation times and concentrations. Lactobacilli spp. produce a class of molecules with antimicrobial capacity (28). For example, bacteriocins produced by L. fermentum can reduce adhesion and cytotoxicity properties of MRSA (29). The CFS of L. salivarius and L. fermentum can inhibit the biofilm formation of S. aureus (30). The complete inhibition of Salmonella growth has been shown in the presence of the CFS from L. johnsonii, L. rhamnosus, L. casei, and L. plantarum (31, 32). Lactobacillus reuteri, L. plantarum, and L. fermentum have been extensively investigated for their probiotic effects on pathogenic bacteria (27, 33-35).
Vahedi-Shahandashti et al. (36) stated that some probiotic lactobacilli culture supernatants had an inhibitory effect on Serratia marcescens swarming and antibiotic resistanc. Hiawy et al. in Iraq showed that the CFS of L. fermentum has inhibitory effects on the growth rate and expression reduction of the gene responsible for biofilm formation in MRSA (21). Our results indicated that L. reuteri, L. fermentum, and L. plantarum reduced the expression of the tst, sae, and sea genes, as well as the agr quorum-sensing system, in MRSA without any tangible effects on its growth. The agr A system is an important global regulatory system that controls the expression of many genes associated with secreting proteins and virulence determinants including protease and collagenase in S. aureus (37, 38). Based on our results and in parallel with the Li et al.’s (39) investigations, it is proven that the reduction in the expression of virulence factors is related to the reduction in the agr system. In fact, the agr A gene is down-regulated at the transcriptional level by CFS. Consequently, it reduces the expression of the sae, tst, sea, sbi, and spa genes. In parallel with other similar studies, it seems that the tst gene is regulated by the sae and agr systems (40).
The results showed that the significant down-regulation of gene expressions was in the preference of the 1/2 × CFS concentration while none of the genes was significantly altered at the low concentration of CFS (1/4 × CFS). It has been proven that the sub-lethal dosage of these antimicrobial compounds can reduce the expression of virulence-associated genes in S. aureus and Vibrio cholera, without affecting their growth (7, 39, 41-43). These results reveal that increasing the incubation time causes a significant reduction in the gene expression so that a significant reduction in the sbi and spa gene expressions achieved by increasing the incubation time. In addition, the CFS of these species of LAB was effective on the level of gene expression. Our findings showed that the CFS of L. reuteri and L. plantarum had a more powerful effect than the CFS of L. fermentum on gene expression. Previous researches suggested that human probiotic isolates, such as L. plantarum, inhibited bacterial virulence, inhibited bacterial virulence, representing a promising alternative to antibiotic prophylaxis of staphylococcal menstrual TSS and potentially of other S .aureus-mediated diseases (40, 44). Though the actual physiological function of supernatant compounds is unclear, these compounds may serve signaling molecules involved in host-bacterial interactions, considering their biological effects in humans (39, 45).
It is necessary to mention that L. fermentum is not recognized as a well-known probiotic bacterium. It had an inhibitory effect on virulence gene expression, depending on concentration (1/2 more effective than 1/4 CFS concentration) and incubation time. It seems that a reduction in gene expression is associated with the pathogenesis and consequently their supernatant component is due to the down-regulation of virulence genes. Therefore, the compounds found in CFS can influence the agr, sae, sea, tst, sbi, and spa genes to reduce expression at the transcription level.
5.1. Conclusions
Probiotic bacteria and their products in food can inhibit the growth and down-regulate virulence gene expression. Consequently, toxins (enterotoxins) and enzymes are less produced by S. aureus as a bacterium causing vomiting. Therefore, the presence of CFS in the food probably reduces diarrhea and vomiting caused by bacteria.