1. Background
The growing demand for natural ingredients for commercial applications, including food, feed, cosmetic, and pharmaceutical applications, is boosted by increased consumers’ demand for more efficient, eco-friendly, and less hazardous products (1-3). Plants, mainly glycophytes, are one of the main sources of natural products for the above-mentioned trades. Halophytes are still less explored, despite their high biotechnological potential (4-8). Halophytes are equipped with several defense mechanisms against stressful environmental factors, in terms of, for example, salinity and temperature, such as the synthesis and accumulation of primary and secondary metabolites, including phenolic compounds. Along with their protective role in plants, these molecules display several biological properties (e.g., antioxidant and anti-inflammatory) with relevant human and animal health benefits and potential commercial applications (9-13).
The Lotus genus (Fabaceae) contains both glycophytic forage crops and those adapted to saline environments (14). Lotus species, such as L. corniculatus L. (birdsfoot trefoil) and L. tenuis Waldst and Kit (narrow-leaf birdsfoot trefoil) are important constituents of grassland ecosystems in saline and desertified areas and are used as forage for livestock due to their high productivity and feeding value (15). Moreover, several Lotus species have traditional medicinal applications; for example, L. corniculatus is used for its anti-inflammatory, antispasmodic, cardiotonic, carminative, febrifuge, hypoglycaemic, and vermifuge properties (16, 17), and displays antiproliferative and antimicrobial activities (16, 18-20). Lotus species contain several groups of bioactive molecules, such as steroids, coumarins, tannins, and flavonoids (21, 22).
Lotus creticus L. (creta trefoil) grows in coastal dunes and sandy shores of the Mediterranean Area. It has a rapid growth rate and low water requirement and is tolerant to salt concentrations up to 100 mM (23). Creta trefoil is considered a major pastoral legume crop in many countries, such as Tunisia, and could be a promising alternative industrial crop for forage purposes in arid and saline areas of the Mediterranean region. Considering the medicinal uses of Fabaceae and Lotus genus, creta trefoil could also be explored as a sustainable source of natural products with industrial applications. However, only a few reports are available describing the chemical composition of creta trefoil roots (24, 25).
2. Objectives
Aiming to fulfill this gap, this work was to unravel the potential use of creta trefoil collected in southern Portugal (Algarve) as a source of bioactive natural products. For this purpose, eco-friendly extracts were obtained by an ultrasound-assisted extraction from aerial parts (stems and leaves) and fruits and evaluated for acute toxicity on mammalian cell lines and polyphenol contents by liquid chromatography coupled with diode array detection and electrospray ionization tandem mass spectrometry (LC-DAD-ESI-MS/MS). Samples were appraised for inhibition capacity on key enzymes in selected pathologies/processes, namely acetyl- and butyryl-cholinesterase (AChE and BuChE, neurodegeneration), α-glucosidase and α-amylase (type 2 diabetes mellitus, T2DM), and tyrosinase (hyperpigmentation/food browning). Extracts were also evaluated for in vitro antioxidant properties.
3. Methods
3.1. Chemicals
Sigma-Aldrich (Germany) supplied 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), flavone, gallic acid, rutin, lipase (EC 3.1.1.3), elastase (EC 3.4.21.36), tyrosinase (EC 1.14.18.1), 4-nitrophenyl dodecanoate (NPD), N-succinyl-ala-ala-alap-nitroanilide (SANA), N-[3-(2-Furyl) acryloyl]-leu-gly-pro-ala (FALGPA), 4- dimethylaminocinnamaldehyde (DMACA), green tea catechin mix, Ginkgo biloba flavonoids mix, and procyanidin B2. Acetonitrile and methanol (HPLC grade) were acquired from VWR (Fontenary-Sous-Bois, France). Ethanol, methanol, and formic acid were provided by Merck (Darmstadt, Germany). Additional solvents and chemicals, including those for cell culture, were delivered by VWR International (Belgium).
3.2. Plant Material
Plants (voucher number XBH43) were sampled in Faro beach, south of Portugal (coordinates: 37°0’0.163” N, -7°9’86.070” W) in July 2018. Plants were divided into aerial vegetative organs, containing stems and leaves, and fruits (pods). Samples were dried in an oven at a temperature of 45°C, for three days, reduced to powder, and stored at -20°C.
3.3. Extraction
Dried biomass was added to ethanol, acetone, and water (1:40, w/w). These solvents were selected because they are allowed in food processing (directive 2009/32/EC of the European Parliament and of the Council of 23 April). Extraction was performed at room temperature (RT, approximately 20°C) for 30 min, by an ultrasound-assisted procedure. The extracts were sieved through Whatman no. 4 paper filters, and the solvents were removed in a rotary evaporator at controlled pressure and temperature. Dried residues were weighted, mixed with methanol at 50 mg/mL, and kept at -20°C in the dark.
3.4. In Vitro Toxicological Evaluation
Two mammalian cell lines were used: Murine RAW 264.7 macrophages (gifted by the Faculty of Pharmacy and Centre for Neurosciences and Cell Biology, University of Coimbra, Portugal) and human embryonic kidney cells (HEK 293) (provided by the Functional Biochemistry and Proteomics, and the Marine Molecular Bioengineering groups, Centre of Marine Sciences, Portugal). Macrophages were cultured in RPMI 1640 media, while embryonic cells were kept in DMEM media, both complemented with 10% heat-inactivated FBS, 1% L-glutamine (2 mM), and 1% penicillin (50 U/mL)/streptomycin (50 µg/mL). General culture conditions were 37°C and moistened atmosphere with 5% CO2.
For the evaluation of cytotoxicity, confluent (80%) cells were plated in 96-well plates at a density of 1 × 104 cells/well (RAW) and 5 × 103 cells/well (HEK) and incubated for 24 h. Cells were then treated for 72 h with the extracts at a concentration of 100 µg/mL. Methanol (0.2%) was applied to control cells. For the determination of cellular viability, the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method was performed (9). Absorbances were read on a microplate reader (EZ read 400, Biochrom), and the results were expressed as cellular viability (%).
3.5. In Vitro Enzymatic Inhibition
In all the assays, the extracts were tested at diverse concentrations (0.5, 1, and 5 mg/mL). The cholinesterase inhibition of the extracts was evaluated by Ellman’s method, according to the method reported elsewhere (26). Galantamine (0.5 - 5 mg/mL) was used as the positive control, and the results were conveyed as equivalents of galantamine (mg GALAE/g extract). The tyrosinase inhibition was also performed according to Zengin (26), using kojic acid as the positive control (at an equal concentration of the extracts). The results were presented as equivalents of kojic acid (mg KAE/g). The amylase and glucosidase inhibition was also evaluated according to Zengin (24). Acarbose (0.5 - 5 mg/mL) was used as a standard, and the results were communicated as equivalents of acarbose (mmol ACAE/g).
3.6. Determination of In Vitro Antioxidant Activities
3.6.1. Radical-based Methods
Radical scavenging activity (RSA) towards free radicals DPPH and ABTS was evaluated in the extracts (10 - 5000 µg/mL) according to Rodrigues, Soszynski (13). The results were conveyed as the percentage of inhibition (%) relative to the negative control and as IC50 (mg/mL) whenever possible. The synthetic antioxidant BHT (butylated hydroxytoluene) was used as the positive control at concentrations amid 10 and 5000 µg/mL.
3.6.2. Metal-based Methods
The extracts were tested at different concentrations (10 - 5000 µg/mL) in all the assays. The metal chelation properties towards copper (CCA) and iron (ICA) were evaluated according to previously described methods (13). The results were expressed as a percentage relative to the negative control and as IC50 (mg/mL) when possible. ethylenediaminetetraacetic acid (EDTA) was used as the positive control. The evaluation of the iron-reducing power (FRAP) was made according to the method reported previously (7). A rise in samples’ absorbance was indicative of an increase in the samples’ reducing properties; thus, the results were expressed as a percentage of inhibition relative to BHT at the concentration of 1 mg/mL, and as IC50 (µg/mL) whenever possible.
3.7. Chemical Profiling of the Extracts by Liquid Chromatography Coupled with Diode Array Detector (LC-DAD-ESI-MS/MS)
The used system comprised an Agilent Technologies 1200 series LC equipped with a diode array detector (DAD), combined with a Bruker Esquire HCT ultra ion trap mass spectrometer (MS) furnished with an electrospray ionization (ESI) source (Agilent). A Hamilton PRP-1 LC column (15.0 cm length, 2.1 mm internal diameter, 5 µm particle diameter), kept at 25°C, was used. The mobile phase was water (A) and acetonitrile (B), with 0.1% of formic acid (v/v). The gradient began with 90% of A, followed by a linear increase until 30% of B in 15.0 min. The mobile phase composition then changed to 100% of B in 15 min and maintained constant for eight minutes. The mobile phase was then permitted to recuperate the initial conditions (90% of A) in 0.5 min and then stabilize for five minutes before the next run. The flow was 0.35 mL/min, and the injection volume was 10 µL. The LC-DAD chromatograms were acquired at 280, 350, 370, and 520 nm, and the spectral data were collected amid 250 and 700 nm. The MS was run in the Auto-MS mode under negative ESI. The spray and ion optics analysis settings were as follows: Capillary voltage, 3.0 kV; Nebulizer gas pressure, 45 psi; Drying gas temperature, 330 °C; Drying gas flow, 8.0 L/min; Capillary exit voltage, 183.0 V; and Skimmer voltage, 60.3 V. Identification was made by relating the UV-V is and fragmentation spectra of compounds detected in samples with authentic standards or data published in the literature. Stock standard solutions were set in ethanol, and working solutions were prepared daily.
3.8. Statistical Analyses
The results were conveyed as mean ± standard error of the mean (SEM), and assays were performed at least three times. Significant differences were evaluated by the analysis of variance (ANOVA), Tukey HSD test, or Kruskal-Wallis test if parametricity did not succeed. Differences were considered significant when P ≤ 0.05. Statistical tests were made using the RStudio (version 1.0.44, 2016). Half-maximal inhibitory concentration (IC50) values were estimated by data sigmoidal fitting (GraphPad Prism V. 5.0 software).
4. Results and Discussion
4.1. In Vitro Toxicological Evaluation
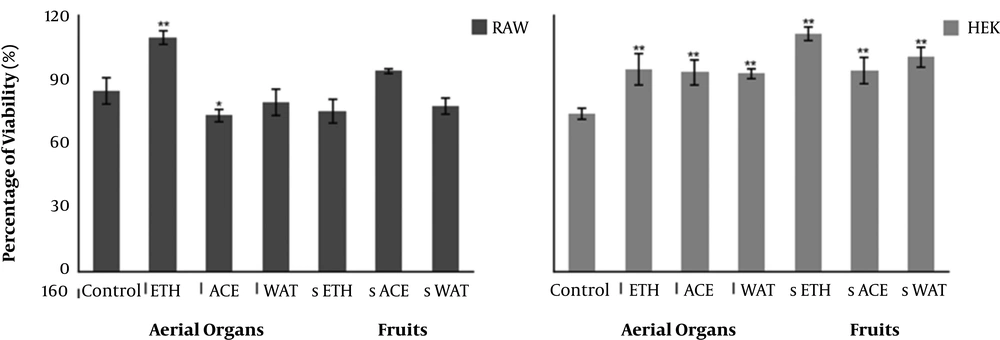
In vitro screening assays for cytotoxicity using mammalian cell lines are recommended to establish the possible toxic properties of natural products (11, 27). Therefore, the effect of the extracts on cellular viability was evaluated on murine RAW 264.7 macrophages and human embryonic (HEK 293) kidney cells. Samples had low toxicity (Figure 1), suggesting that we could proceed with the evaluation of biological properties. However, the safety of these extracts must be confirmed by complementary toxicity models, such as Artemia salina.
Effect of the application of 100 µg/mL of different extracts of creta trefoil (L. creticus) on RAW 264.7 and HEK 293 cellular viability. Results are expressed as % of viability relative to the negative control. Solid and error bars represent the average and SEM, respectively (n = 12). Significant differences between positive control and treated cells are indicated as follows; *, P < 0.05; **, P < 0.005. ETH, ethanol extracts; ACE, acetone extracts; WAT, water extracts.
4.2. In Vitro Enzymatic Inhibition
The progression of Alzheimer’s disease (AD) is linked to a significant reduction in the neurotransmitter acetylcholine (ACh), which is responsible for memory and cognition (28). Therefore, maintaining a normal level of ACh by inhibiting its main hydrolyzing-enzyme (AChE), and also BuChE, is considered as the main therapeutic approach to manage AD symptoms (29, 30). The use of cholinesterase inhibitors is also reported as a promising adjunctive therapy in depressive and schizophrenia situations (31, 32). Except for ethanol leaves extracts, all samples inhibited AChE, especially acetone extracts from the aerial organs (1.28 mg GALAE/g) and fruits (1.07 mg GALAE/g) (Figure 1). Excluding the water leaves extracts, all samples inhibited BuChE, especially ethanol (1.30 mg GALAE/g) and acetone (1.24 mg GALAE/g) fruit extracts (Figure 1). As far as we know, there is no information of possible neuroprotective properties of Lotus species, although other legumes, such as Medicago sativa L, have been reported with AChE inhibitory activity (33). Our results suggest that creta trefoil should be further explored as a source of neuroprotective agents.
Products with the capacity to inhibit α-glucosidase and α-amylase can dangle the breakdown of carbohydrates and reduce the postprandial upsurge of blood glucose level that results from a varied carbohydrate meal, and thus, are important for the management of hyperglycemia linked to T2DM (29, 34). The extracts displayed moderate inhibition on α-glucosidase (approx. 2 mmol ACAE/g), and a low capacity to inhibit amylase (from 0.03 to 0.28 mmol ACAE/g). These results suggest that additional studies could be pursued with creta trefoil aiming to clarify its hypoglycemic properties, especially because products with slight amylase inhibitory activity and potent glucosidase inhibitory action are desired (35).
Samples were further evaluated for their capacity to inhibit tyrosinase, which is involved in three steps of melanin synthesis: Hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA), oxidation of DOPA to DOPA, and oxidation of 5,6-dihydroxyindole to indolequinone (36). Tyrosinase is also involved in the oxidation of phenolic compounds to quinones, and therefore, in the enzymatic browning of vegetable products (37), and is involved in diverse critical processes in insects, such as melanogenesis, parasite encapsulation, and sclerotization (38, 39). As can be seen in Table 1, the potent inhibition of tyrosinase was observed after treatment with all the samples, except for water leaves extracts. The best results were observed with acetone (28.20 mg KAE/g) and water (23.53 mg KAE/g) extracts from fruits, and ethanol (27.84 mg KAE/g) and acetone (27.01 mg KAE/g) extracts from leaves. To the best of our knowledge, there are no reports of tyrosinase inhibition by creta trefoil. In a previous report, an 80% aqueous ethanol extract of whole L. corniculatus plant had no activity towards tyrosinase (40). Our results suggest that creta trefoil could be explored as a source of tyrosinase inhibitors with potential use as, for example, pharmaceuticals, to prevent or treat hyperpigmentation disorders (e.g. melasma), food preservatives, to prevent the undesirable browning of plant-derived foods, and in agriculture, to control insect pests (36, 37, 39).
| Samples | AChE, mg GALAE/g | BChE, mg GALAE/g | Tyrosinase, mg KAE/g | Amylase, mmol ACAE/g | Glucosidase, mmol ACAE/g |
|---|---|---|---|---|---|
| Aerial organs | |||||
| Ethanol | NA | 1.07 ± 0.09B | 27.84 ± 0.34A | 0.20 ± 0.01B | 2.23 ± 0.01A |
| Acetone | 1.28 ± 0.03A | 1.11 ± 0.06B | 27.01 ± 0.46A | 0.39 ± 0.01A | 1.62 ± 0.23B |
| Water | 0.69 ± 0.02C | NA | 7.79 ± 0.34C | 0.02 ± 0.01 | 0.41 ± 0.01C |
| Fruits | |||||
| Ethanol | 0.70 ± 0.01C | 1.30 ± 0.01A | 12.00 ± 0.01 | 0.24 ± 0.03B | 0.41 ± 0.01C |
| Acetone | 1.07 ± 0.33B | 1.24 ± 0.05A | 28.20 ± 0.89A | 0.28 ± 0.01B | 2.13 ± 0.04A |
| Water | 0.98 ± 0.03B | 0.16 ± 0.08C | 23.53 ± 0.24B | 0.03 ± 0.02C | 2.12 ± 0.02A |
Abbreviation: NA, no activity.
aIn the same column, values followed by the same capital letters (A-C) are not significantly different referring to the Tukey HSD test (P < 0.05).
bValues are expressed as mean ± standard error of the mean (SEM) of at least three experiments made in triplicate (n = 9).
4.3. In Vitro Antioxidant Activity
Antioxidants are an important group of molecules from different types, such as phenolics, which can reduce the negative effects of free radicals and oxidative damage (41-43). The use of natural antioxidants can therefore increase the body’s resistance to oxidative injuries and have a significant impact on human and animal health (44, 45). Natural antioxidants also have possible applications as food preservatives and additives to replace synthetic antioxidants, such as BHT (40). In this work, creta trefoil extracts were evaluated for in vitro antioxidant properties, and the results are summarized in Table 2.
| Samples | DPPH | ABTS | CCA | ICA | FRAP |
|---|---|---|---|---|---|
| Aerial organs | |||||
| Ethanol | 0.91 ± 0.0C | 1.07 ± 0.16C | NR | 0.36 ± 0.06B | |
| Acetone | 4.79 ± 0.21D | 1.82 ± 0.13C | 5.50 ± 0.0D | NR | 0.33 ± 0.04B |
| Water | NR | 5.67 ± 0.70D | NR | 3.09 ± 0.35 | 1.32 ± 0.09D |
| Fruits | |||||
| Ethanol | 0.25 ± 0.0B | 0.41 ± 0.1B | 1.51 ± 0.0AB | NR | 0.22 ± 0.02B |
| Acetone | 4.32 ± 0.76D | 3.06 ± 0.0D | 1.15 ± 0.18B | NR | 0.41 ± 0.02B |
| Water | 0.44 ± 0.01B | 1.89 ± 0.19C | 3.20 ± 0.15C | NR | 0.53 ± 0.01BC |
| BHTd | 0.1 ± 0.02A | 0.06 ± 0.0A | 3.48 ± 0.22C | NR | |
| EDTAd | 0.11 ± 0.0A |
Abbreviation: NR, IC50 value was not reached.
aIn the same column, values followed by the same capital letters (A-D) are not significantly different referring to the Tukey HSD test (P < 0,05).
bThe results represent IC50 values (mg/mL).
cValues are expressed as mean ± standard error of the mean (SEM) of at least three tests, in triplicate (n = 9).
dPositive controls.
Fruit extracts exhibited, in general, a higher antioxidant capacity. Fruit ethanol extracts had the highest DPPH RSA (IC50 = 0.25 mg/mL) and FRAP (IC50 = 0.22 mg/mL). Regarding aerial vegetative organs, the ethanol and acetone extracts had the highest FRAP, with IC50 values of 0.36 and 0.33 mg/mL, respectively. The ethanol extracts also displayed RSA against DPPH, with IC50 values of 0.91 and 1.07 mg/mL, correspondingly. In the reviewed literature, we did not find reports on the antioxidant properties of creta trefoil. However, extracts from other Lotus species exhibited relevant in vitro antioxidant properties; for example, a methanol extract of L. corniculatus was active in the β-carotene/linoleic acid assay, while a butanol extract of the same species bore good results in the DPPH, H2O2 scavenging, and BSA denaturation assays, as well as in an in vivo (paracetamol-induced hepatitis in rats) study (17, 46). Our results suggest that creta trefoil could be further explored as a source of natural antioxidants with potential health improvement purposes, to mitigate oxidative stress-related disorders, or as antioxidant food preservatives (45, 47, 48). Moreover, having in mind the possible use of creta trefoil for forage purposes (23), the presence of antioxidant compounds in this species could suggest its role to prevent oxidative stress-related disorders, such as those affecting animal reproduction, which causes severe loss to the livestock industry (45). The antioxidant properties of creta trefoil, targeting not only animal but also human health, should be further explored by additional in vitro assays (e.g., cell-based methods) and in vivo studies.
4.4. Chemical Characterization
Lotus species are rich in polyphenols, such as flavonoids (21, 49, 50). In this work, the major flavonoid compounds present in the extracts were identified, and their relative contents were estimated by LC-MS (Table 3).
| Samples Number | RT | [M-H]- | Assignment | Major MS2 Signals and Relative Intensity | Fruits | Leaves | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Acetone | Water | Ethanol | Acetone | Water | |||||
| Flavonols | ||||||||||
| 1 | 10.8 | 625 | Quercetin-O-dihexoside | 463 (100), 301 (32) | ++ | + | +++ | + | + | ++ |
| 2 | 11.4 | 625 | Quercetin-O-dihexoside | 463 (189), 301 (29) | ++ | - | +++ | - | - | + |
| 3 | 16.8 | 479 | Myricetin-O-hexoside | 317 (98), 316 (100) | +++ | + | ++ | - | - | - |
| 4 | 17 | 479 | Myricetin-O-hexoside | 317 (100), 316 (100) | +++ | + | ++ | - | - | ++ |
| 5 | 19.2 | 463 | Quercetin-O-hexoside | 301 (100) | ++ | + | +++ | + | + | + |
| 6 | 19.4 | 463 | Quercetin-O-hexoside | 301 (100) | +++ | + | ++ | - | - | + |
| 7 | 20.4 | 477 | Isorhamnetin-O-hexoside | 315 (189), 314 (100) | +++ | ++ | +++ | ++ | + | ++ |
| 8 | 23.7 | 301 | Quercetin | 273 (17), 257 (21), 179 (100), 151 (67) | +++ | ++ | + | + | + | + |
| 9 | 25.8 | 315 | Isorhamnetin | 300 (100) | +++ | ++ | + | - | - | - |
| Catechins and Catechin Oligomers | ||||||||||
| 10 | 6.9 | 305 | Gallocatechin | 221 (100), 219 (79), 179 (95), 261 (23), 287 (18), 165 (23) | ++ | + | - | - | - | - |
| 11 | 10 | 305 | Epigallocatechin | 265 (100), 219 (62), 137 (54) | ++ | + | - | - | - | - |
| 12 | 11.3 | 289 | Catechin | 245 (100), 205 (31), 203 (22), 179 (10) | +++ | ++ | + | - | - | - |
| 13 | 13.9 | 289 | Epicatechin | 245 (100), 205 (31), 203 (22), 179 (10) | +++ | ++ | - | - | - | - |
| 14 | 12.1 | 457 | Gallocatechin gallate | 427 (100), 349 (88), 317 (69), 193 (56), 126 (75) | ++ | + | +++ | ++ | + | +++ |
| 15 | 12.8 | 577 | Procyanidin B2 | 407 (100), 451 (36), 425 (44), 559 (12), 289 (35), 145 (7) | +++ | - | - | - | - | - |
Abbreviation: RT, retention times.
aIdentification performed using authentic standards or based on references (Xiao et al., 2012; Francescato et al., 2013).
bThe relative amount of compounds between extracts were labeled +, ++, or +++ for low, medium, and high peak areas, respectively.
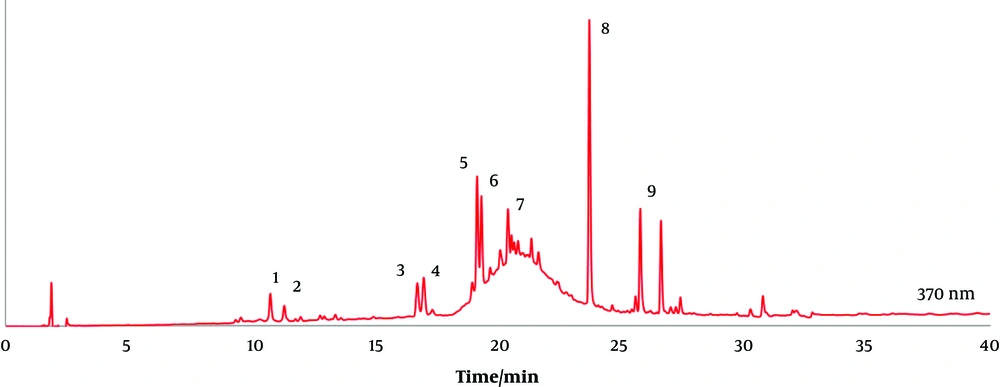
Main flavonoids included flavonols, catechins, and catechin oligomers (Table 3 and Figure 2). Fruits had the highest diversity of such compounds, especially the ethanol and water extracts (Table 3). Quercetin and its derivatives, namely quercetin-O-dihexoside and isorhamnetin-O-hexoside, were present in all samples, especially the ethanol fruit extracts (Table 3). Quercetin is an important flavonoid present in many plant species, including glycophytes (such as Camellia sinensis (L.) Kuntze, and halophytes, including Polygonum maritimum L. (42, 43)) (43). Quercetin displays strong antioxidant properties (51-54). Therefore, the higher antioxidant activity displayed by the fruit extracts, particularly the ethanolic extract, should be correlated with quercetin and quercetin derivatives (Tables 1 and 3). Quercetin is also likely involved in the detected enzymatic inhibitory properties, as it is known that it can inhibit cholinesterases (55, 56), α-glucosidase, α-amylase (57), and tyrosinase (58).
The LC-DAD profile at 370 nm of an ethanol extract of creta trefoil (L. creticus) leaves. Assignments; 1, quercetin-O-dihexoside; 2, quercetin-O-dihexoside; 3, myricetin-O-hexoside; 4, yricetin-O-hexoside; 5, quercetin-O-hexoside; 6, quercetin-O-hexoside; 7, isorhamnetin-O-hexoside; 8, quercetin; 9, isorhamnetin (see Table 3).
Other identified flavonols included myricetin derivatives (Table 3). Myricetin is a common plant-derived flavonoid in many fruits, vegetables, and teas (59), and is present in some halophyte species, such as P. maritimum (60). Myricetin displays several biological activities similar to quercetin, including antioxidant activity (59, 61) and enzymatic inhibition towards cholinesterases, α-glucosidase, and tyrosinase (39, 57, 62), which should also be related to the biological properties detected in the creta trefoil extracts. Catechins and catechin oligomers were also identified, particularly in the ethanol and aqueous extracts (Table 3 and Figure 3), as observed for other Lotus species (e.g. L. tenuis (cv. Larrañaga) and L. corniculatus) (22, 63). Catechins are natural polyphenolic compounds (-flavan-3-ols of the flavonoid family) and are abundant in several plant products, especially green tea (64). These compounds are strong antioxidants (64), which may be related to the antioxidant activity observed in the creta trefoil extracts. Moreover, catechin derivatives such as gallocatechin and (+)-catechin-aldehyde polycondensates display enzymatic inhibitory properties (50, 51), which may help explain some of the bioactivities detected in this work.
To the best of our knowledge, this is the first report of the existence of the above-mentioned compounds in creta trefoil. In previous work, the flavanone euchrestaflavanone A (3) and three isoflavonoids, namely, luprnalbin A-C and 4’-methyl ether of wighteone, were identified in an aqueous ethanol extract of the roots of the same species (25). In the petrol and alcoholic extract of the roots of L. creticus were identified sitosterol, sitosterol B-ursolic acid, linamarin, lotaustralin, and a coumestan derivative (24).
4.5. Conclusions
Creta trefoil extracts showed low toxicity in vitro towards two mammal cell lines. The fruit extracts had the highest antioxidant capacity, especially ethanolic extracts. The ethanol and acetone extracts from stems and leaves also had a high capacity to reduce iron and scavenge DPPH. Except for the ethanol leaves’ extracts, all samples could inhibit AChE, especially acetone samples from the aerial vegetative organs. Excluding the water leaves’ extracts, all samples were able to inhibit BuChE, especially the ethanol and acetone fruit samples. The acetone and water extracts from fruits and the ethanol extract from leaves had moderate inhibition on α-glucosidase and low capacity to inhibit α-amylase. Samples had, in general, potent tyrosinase inhibition. The extracts were mainly composed of flavonols, catechins, and catechin oligomers, and fruits had the highest diversity of the detected compounds. Altogether, these results indicate that creta trefoil may be a promising source of bioactive products and molecules with multiple applications, such as, for example, in the food/feed, pharmaceutical, cosmetic, and agriculture areas.