1. Background
Cinnamon, as one of the most popular spices worldwide, is widely used in cooking as well as an effective substance in traditional and supplementary medicine (1, 2). Producing the aroma and many biological properties of the compound are due to cinnamaldehyde and trans-cinnamaldehyde that are considered the most important constituents of cinnamon (3).
Diabetes is one of the most common non-communicable diseases often associated with many complications in patients. The processes of absorption, distribution, and metabolism of drugs in diabetic patients are changed as the result of vascular complications (4). This leads to intensive inter-personal changes in drug plasma concentrations. It seems that changes in drug-metabolizing enzymes in the liver highly influence plasma concentrations.
The CYP450 enzyme family includes the most significant enzymes involving in the phase I reactions. To date, several studies have demonstrated changes in the expression level of cytochrome P450 in type 1 and 2 diabetes (5, 6). However, changes in the activity of this enzyme family and hepatic clearance have not been specifically studied in type 1 and 2 diabetic patients. Given the significant roles of these enzymes in the metabolism of commonly used drugs as well as the importance of metabolic changes in the development of inter-personal variations in medication, it seems necessary to understand the activity of liver CYP450 enzymes in these patients.
CYP2D6 is involved in the metabolism of at least 20% of clinically presented drugs, including β-blockers, anti-cancer medicines, antiarrhythmics, antipsychotics, tricyclic antidepressants and also, selective estrogen receptor modifier, opioid analgesics, like codeine, tramadol, etc. (7, 8). Therefore, considering the high prevalence of diabetes mellitus, examining the alteration in the activity of this enzyme and assessing the effect of cinnamon treatment on enzymatic performance is of high importance.
In many countries, cinnamon is ordinarily used as a traditional treatment to decrease blood sugar levels in patients with diabetes mellitus (9). CYP2D1 has been shown to be the rat orthologue of human CYP2D6 and is a good alternative for assessing the activity of this enzyme in animal models (10). Based on the previously mentioned remarks, in this study, we examined the effects of type 1 and type 2 diabetes on CYP2D1 activity in an animal model of diabetes mellitus. Because streptozotocin (STZ)-induced diabetes is widely used in animal models in pharmacokinetic studies (11, 12), we selected the type 1 (using STZ) and type 2 (using STZ -nicotinamide) diabetic rat models for the present study. On the other hand, perfusion of the liver has been applied widely in toxicological and pharmacological research in the last years (13). Changes in the ratio of dextrorphan (DXO) to dextromethorphan (DXM) has been accepted as a probe for assessing the rat CYP2D1 activity (14).
It should be noted that the two most important types of diabetes were selected to investigate the effect of acute and chronic inflammatory conditions on the activity of CYP enzymes. In this study, any changes in the metabolic ratio of DXM were considered as an index to evaluate the effects of cinnamon consumption with or without routine treatments of both types of diabetes on the CYP2D1 activity and the hepatic clearance parameters in a rat perfused liver model.
2. Methods
2.1. Materials and Reagents
STZ (Zanosar®) was obtained from Sigma- Aldrich (St.Louis, MO). Nicotinamide was obtained from Merck Co. (Darmstadt, Germany). Cinnamon (Cinnamomum verum) powder of bark was purchased from Roha Arzneimittel GmbH (Bremen, Germany). DXM and its two metabolites, N-demethylated dextromethorphan, and O-demethylated dextromethorphan as internal standard were purchased from the department of medicinal chemistry, School of Pharmacy, Tehran University of Medical Science (Tehran, Iran), and structural confirmation of the compounds were conducted by C-NMR and H-NMR in the respective laboratory. The acetonitrile and methanol (high-performance liquid chromatography (HPLC) grade) were purchased from Duksan Pure Chemicals Co.Ltd (Gyeonggi-do, Korea). Other chemicals with analytical-grade were obtained from Merck (Darmstadt, Germany).
2.2. Animals
Thirty-two male Sprague-Dawley rats with normal weight (220-250 g) were purchased from Animal Laboratory, Tehran University of Medical Science (Tehran, Iran). Housing conditions were kept in standard conditions (12-h day/night cycle and free access to rodent chow and water) for all rats. In order to induce type 1 diabetes mellitus, a single (I.P.) dose of STZ (Sigma, USA) with a concentration of 65 mg/kg was freshly dissolved in 100 mM sodium citrate buffer at pH 4.5 and intraperitoneally injected to overnight fasted rats (Kamei et al., 2005). Also, in order to induce type 2 diabetes, after 12 hours of fasting, firstly, 110 mg/kg Nicotinamide (dissolved in normal saline) was intraperitoneally injected. Then, 65 mg/kg Streptozocin (dissolved in citrate buffer; pH = 4.5) was intraperitoneally injected after 15 minutes. Measuring fasting blood sugar (FBS) level using a kit (Jiancheng Bioengineering Ins. China) confirmed induction of diabetes. Rats with the measured FBS of more than 200 mg/dL and 400 mg/dL following STZ plus Nicotinamide and STZ administration were considered as type 2 and type 1 diabetic rats. All rats were randomly divided into eight groups of 4 rats: Group 1: Control group of healthy and normal rats; Group 2: Uncontrolled type 1 diabetic rats without any treatment; Group 3: Type 1 diabetic rats receiving cinnamon; Group 4: Type 1 diabetic rats receiving cinnamon plus insulin; Group 5: Uncontrolled type 2 diabetic rats without any treatment; Group 6: Type 2 diabetic rats receiving cinnamon; Group 7: Type 2 diabetic rats receiving cinnamon plus metformin; Group 8: Healthy and normal rats receiving cinnamon.
Non-diabetic normal rats (group 1) and uncontrolled type 1 and 2 diabetic rats (group 2) only received normal saline solution. After 2 weeks of diabetes induction, 300 mg/kg cinnamon (dissolved in 4 ml normal saline) was administered to cinnamon-receiving rats by gavage for two weeks. The dose of cinnamon was selected based on previous studies on diabetic rats (15, 16). In addition, group 4 was given 6 - 8 IU of isophane insulin by subcutaneous administration (10 IU/mL, Exir Pharmaceutical Co., Tehran, Iran) per day for 14 days. In metformin recipients (group 7), 200 mg/kg of metformin powder dissolved in 4 ml of normal saline (NS) was administrated (by gavage) once a day for 14 days after induction of diabetes mellitus. Then, after 28 days of diabetes mellitus induction and relevant treatment, perfusion of rat liver (IRLP model) was applied to examine the hepatic CYP2D1 enzyme activity and hepatic clearance.
The study protocol was approved by the Institutional Review Board of Pharmaceutical Research Centre, Tehran University of Medical Sciences [IR.TUMS.PSRC.REC.1396.3038].
2.3. The Isolated Liver Perfusion Study
The rats were anesthetized through intraperitoneal injection of the 1:4 mixture of xylazine 2% and ketamine 10%. For each rat, the portal vein and superior vena cava were cannulated as input and output of the perfusion medium, respectively, to perfuse the isolated liver. The bile duct and inferior vena cava were blocked. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were ceaselessly observed and examined by a spectrophotometric method as an index for liver viability and activity (17). The perfusion medium was freshly prepared using Krebs-Henseleit buffer comprising DXM (300 μM) as a probe. The superior vena cava was considered as the outlet duct for sampling, and the perfused samples (1 mL) were collected immediately after washing and then every 5 minutes for the first 30 minutes and every 10 minutes for the second 30 minutes. After centrifuging at the rate of 10000 rpm for 10 min, samples were stored at -70°C until analysis.
2.4. Apparatus and Chromatographic Condition
For assessing the chromatographic condition, a low-pressure gradient HPLC pump with a fluorescence detector (320 excitation- 285 emission nm), a 100 μlit loop, and a Rheodyne 7725i injector, all from Knauer (Berlin, Germany) were used. A ChromolithTM Performance RP-18e 100 mm × 4.6 columns with ChromolithTM guard cartridge RP- 18e 5 mm × 4.6 mm were applied for chromatographic separation (Merck, Darmstadt, Germany). A mixture of 50% KH2PO4 buffer, 30% acetonitrile, and 20% methanol was used as the mobile phase of the procedure, and the flow rate was 1 ml/min. Data acquisition and analyses were done using ChromGate chromatography software (Knauer, Berlin, Germany). The retention time of DXM and DXO was measured to be 10 and 5.5 min, respectively (18).
2.5. Pharmacokinetic Parameters
The area under the curve (AUC0) (0 - 60) was computed by the trapezoidal rule based on concentrations of DXO and its metabolite that has been obtained by mentioned HPLC coupled with fluorescence (FL) detection method.
The mean concentration of each compound was calculated at 40, 50, and 60 minutes of liver perfusion technique. Values obtained were used to determine the mean concentration of DXO and its metabolite (metabolic ratio) at the steady-state in order to evaluate CYP2D1 enzyme activity.
2.6. Statistical Analysis
In the present study, all data were expressed as mean ± SEM. Differences between mean values of groups (P-value < 0.05) were determined by an unpaired t-test.
3. Results
3.1. Effects of Type 1 Diabetes on CYP2D1 Activity
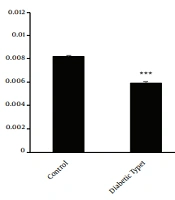
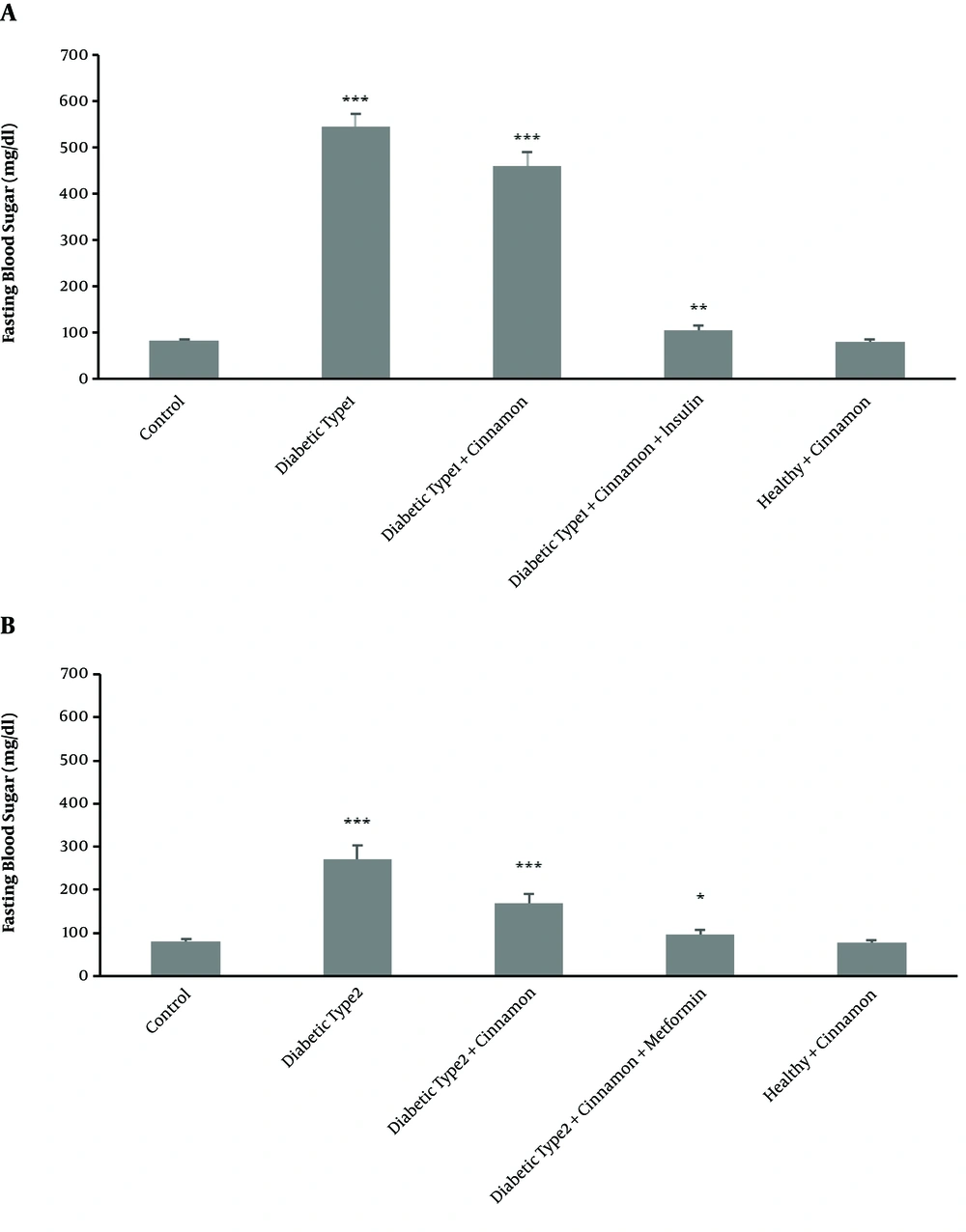
Type 1 diabetes mellitus significantly reduced CYP2D1 activity. Consequently, the mean metabolic ratio of DXO changed from 0.0081 ± 0.00009 in the control group to 0.0059 ± 0.0001 in the untreated type 1 diabetic rats (P-value = 0.00001) (Figure 1). In type 1 diabetic rats, FBS significantly (P-value = 0.00007) increased (543 ± 27 mg/dL) compared with the control group (81 ± 3 mg/dL) (Figure 2A).
Perfused means metabolic ratio profile (3 endpoints) in all type 1 diabetic classes than the control rats after 300 μM dextromethorphan-containing perfusion buffer passage through the portal vein. There are 4 rats in each group. In triplicate experiments, each experiment was replicated independently three times, and the data are shown as mean ± SEM. *P ≤ 0.05; ***P ≤ 0.001.
Fasting Blood Sugar (mg/dL) level compared with untreated type 1 diabetic rats and the insulin-receiving groups (A). Fasting Blood Sugar (mg/dl) level compared with untreated type 2 diabetic rats and metformin receiving groups (B). In triplicate experiments, each experiment was replicated independently three times and the data are shown as mean ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
3.2. Effects of Cinnamon Administration on CYP2D1 Activity in Type 1 Diabetic Rats
Cinnamon administration could not modulate the enzyme activity to the observed level in the control group (P-value = 0.0013). Following two weeks of cinnamon treatment, however, a significant increase (P-value = 0.0023) was observed in enzyme activity in the treated group in comparison with the untreated type 1 diabetic rats. In untreated type 1 diabetic rats, the metabolic ratio changed from 0.0059 ± 0.0001 to 0.0069 ± 0.0005 (Figure 1). The FBS in the cinnamon-receiving diabetic group (460 ± 30 mg/dL) slightly decreased in comparison with untreated group (543 ± 27 mg/dL) (P-value = 0.0065) (Figure 2A).
3.3. Effects of Simultaneous Administration of Cinnamon and Insulin on CYP2D1 Activity in Type 1 Diabetic Rats
Following two weeks of simultaneous administration of cinnamon and insulin, a surprisingly significant decrease (P-value = 0.00002) in CYP2D1 enzyme activity (0.0039 ± 0.00006) was observed in the treated group compared with the control group (0.0081 ± 0.00009) and even untreated type 1 diabetic rats (0.0059 ± 0.0001) (Figure 1). However, the FBS significantly decreased in the diabetic group receiving cinnamon plus insulin (104 ± 10 mg/dL) in comparison with the untreated group (543 ± 27 mg/dL) (P-value = 0.00009) (Figure 2A).
3.4. Effects of Type 1 Diabetes Mellitus on Hepatic Clearance
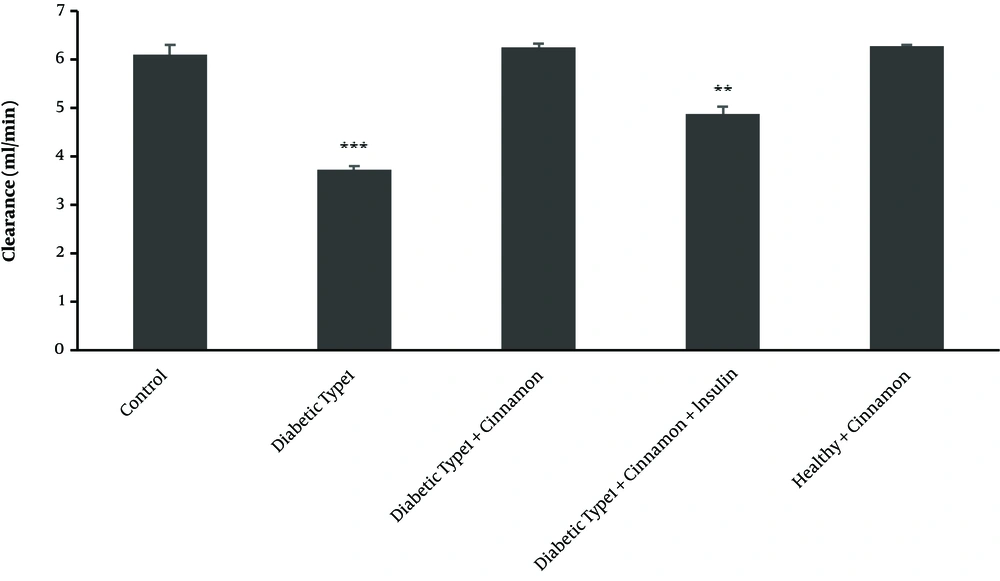
DXO hepatic clearance followed by hepatic perfusion of 300 μM DXO in different studied groups demonstrated decreased hepatic clearance in type 1 diabetic rats (CL = 3.71 ± 0.07 ml/min) compared with the control group (CL = 6.09 ± 0.2 mL/min) (P-value = 0.00009) (Figure 3).
Clearance profile (based on 3 endpoints of dextromethorphan concentrations) in all type 1 diabetic classes than control rats after 300 μM dextromethorphan-containing perfusion buffer passage through the portal vein in triplicate experiments, each experiment was replicated independently three times and the data are shown as mean ± SEM. **P ≤ 0.01; ***P ≤ 0.001.
3.5. Effects of Treatment with Cinnamon on Hepatic Clearance in Type 1 Diabetic Rats
In comparison with the untreated diabetic group (P-value = 0.00008), hepatic clearance increased in cinnamon-receiving group (CL = 6.26 ± 0.06 mL/min), reaching the observed level in the control group (CL = 6.09 ± 0.2 mL/min) (P-value = 0.11) (Figure 3).
3.6. Effects of Simultaneous Treatment with Cinnamon and Insulin on Hepatic Clearance in Type 1 Diabetic Rats
In the group receiving cinnamon plus insulin, DXO hepatic clearance followed by hepatic perfusion of 300 μM DXO indicated the increased hepatic clearance (CL = 4.88 ± 0.13 ml/min) compared with the untreated type 1 group (CL = 3.71 ± 0.07 mL/min) (P-value = 0.00009) (Figure 3).
3.7. Effects of Type 2 Diabetes on CYP2D1 Activity
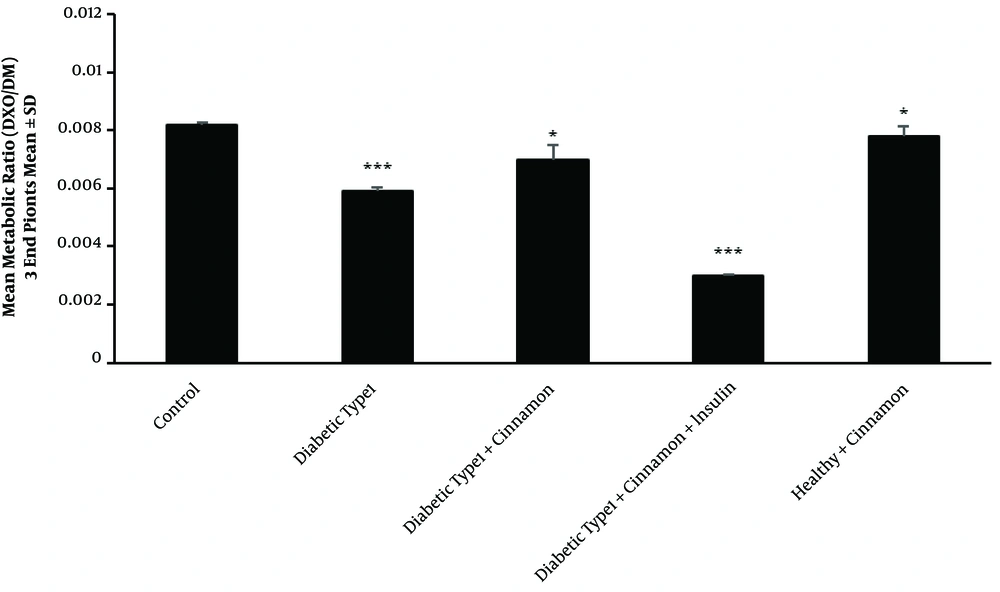
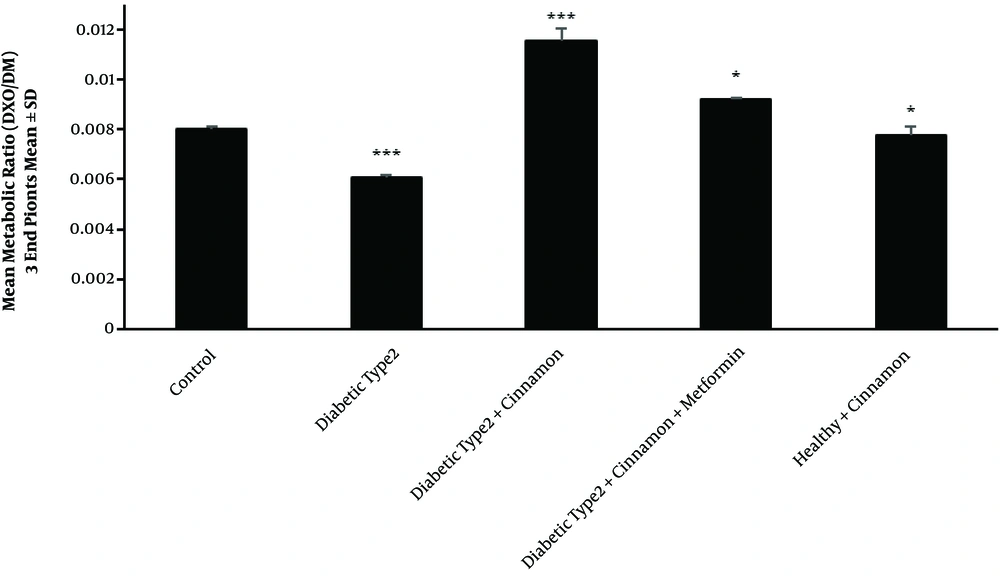
Type 2 diabetes mellitus significantly reduced CYP2D1 activity, resulting in changing the mean metabolic ratio of DXO from 0.0081 ± 0.00009 in the healthy group (control) to 0.006 ± 0.0001 in the untreated type 2 diabetic rats (P-value = 0.00002) (Figure 4). As expected, FBS significantly increased (288 ± 29 mg/dL) (P-value = 0.00006) in the untreated rats compared with the healthy group (81 ± 3 mg/dL) (Figure 2B).
Perfused means metabolic ratio profile (3 endpoints) than the control rats in all type 2 diabetic groups after the passage of a 300 μM dextromethorphan-containing perfusion buffer via the portal vein. There are 4 rats in each group. In triplicate experiments, each experiment was replicated independently three times, and the data are shown as mean ± SEM. *P ≤ 0.05; ***P ≤ 0.001.
3.8. Effects of Cinnamon Administration on CYP2D1 Activity in Type 2 Diabetic Rats
Cinnamon administration increased CYP2D1 activity in the treated group (0.0115 ± 0.0003) compared with the untreated type 2 diabetic rats (0.006 ± 0.0001) (P-value = 0.00001) (Figure 4). In cinnamon-receiving type 2 diabetic group, the FBS slightly decreased (230 ± 27 mg/dL) compared with the untreated group (288 ± 29 mg/dL). This modulation was statistically significant (P-value = 0.0024) (Figure 2B).
3.9. Effects of Simultaneous Administration of Cinnamon and Metformin on CYP2D1 Activity in Type 2 Diabetic Rats
Following two weeks of simultaneous cinnamon and metformin administration, CYP2D1 enzyme activity increased inordinately (P-value = 0.00003) (0.0092 ± 0.0005) in comparison with the untreated type 2 diabetic rats (0.006 ± 0.0001) (Figure 4). In the type 2 diabetic group receiving cinnamon plus metformin, FBS significantly decreased (99 ± 2 mg/dL) in comparison with the untreated group (288 ± 29 mg/dL) (P-value = 0.00002) (Figure 2B).
3.10. Effects of Type 2 Diabetes Mellitus on Hepatic Clearance
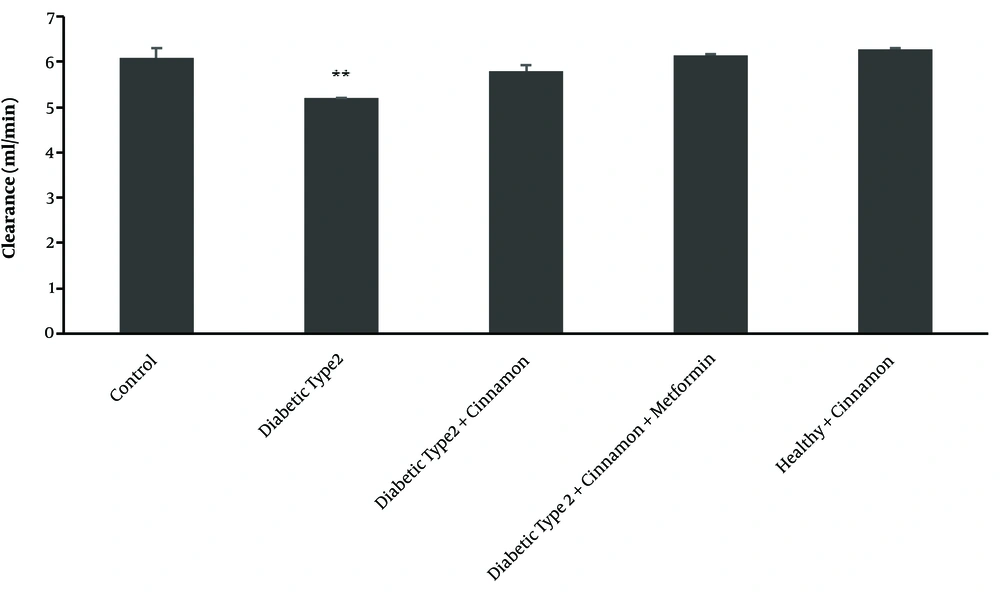
DXO hepatic clearance followed by hepatic perfusion of 300 μM DXO in various groups of the study demonstrated a decrease in hepatic clearance in type 2 diabetic rats (CL = 5.19 ± 0.02 mL/min) compared with the control group (CL = 6.09 ± 0.2 mL/min) (P-value = 0.00003) (Figure 5).
Clearance profile (based on 3 endpoints of dextromethorphan concentrations) for all type 2 diabetic classes than the control rats after the 300 μM dextromethorphan-containing perfusion buffer passes through the portal vein. There are 4 rats in each group. The data are shown as mean ± SEM. **P ≤ 0.01.
3.11. Effects of Treatment with Cinnamon on Hepatic Clearance in Type 2 Diabetic Rats
Hepatic clearance increased in the cinnamon-receiving group (CL = 5.79 ± 0.11 mL/min) compared with the untreated type 2 diabetic group (P-value = 0.00008). However, this increase was not the same as its level in the control group (P-value = 0.0202) (Figure 5).
3.12. Effects of Simultaneous Treatment with Cinnamon and Metformin on Hepatic Clearance in Type 2 Diabetic Rats
In the group receiving cinnamon plus metformin, DXO hepatic clearance followed by hepatic perfusion of 300 μM showed an increase in hepatic clearance (CL = 6.13 ± 0.01 ml/min) compared with the untreated type 2 diabetic group (CL = 5.19 ± 0.02 mL/min) (P-value = 0.0000). The increment was the same as its level in the control group (P-value = 0.634) (Figure 5).
3.13. Effects of Cinnamon Administration on CYP2D1 Activity and Hepatic Clearance in Healthy Rats
In order to investigate the possible effects of cinnamon administration on the enzyme activity in healthy rats, another group was added to the study, in which healthy rats received cinnamon for 14 days.
In the healthy rats receiving cinnamon, enzyme activity decreased slightly (0.0077 ± 0.0003) compared with the control group (0.0081 ± 0.00009) (P-value = 0.037) (Figure 1 and 4). Although hepatic clearance also increased in the mentioned group (6.26 ± 0.04 mL/min) compared with the control group (6.09 ± 0.2 mL/min), this increment was not statistically significant (P-value = 0.0979) (Figure 3 and 5). The FBS of healthy rats receiving cinnamon (78 ± 4 mg/dL) was not statistically different (P-value = 0.4001) from the control group (81 ± 3 mg/dl) (Figure 2).
4. Discussion
In modern medicine, the majority of patients take different medicines every day to treat one or more diseases (19). The basic effect of these drug combinations has been an increased number of drug-drug interactions (20). Many diseases cause one or more physiological and biochemical changes in the absorption, distribution, metabolism, and elimination (ADME) of medicinal products, especially those involved in inflammatory processes (21). The impact of chronic inflammatory diseases, such as diabetes mellitus, on the biotransformation ability of diabetic animals and patients with diabetes mellitus have been tested in multiple studies (22-24).
The drug metabolism process occurs predominantly in the liver, followed by the intestine and kidney, as well as in some other organs with less important roles (e.g., blood, skin, and brain) (25, 26). Drug-metabolizing enzymes are classified into two classes of phase 1 and phase 2 enzymes (27), according to the type of biotransformation undertaken. The family of cytochrome P450 (CYP) enzymes is considered to be one of the most important typical oxidative enzymes in phase 1. In most cases of marketed products, the CYP family of enzymes serves/acts as the predominant catalysts in the biotransformation pathway or removal mechanism (28).
Different diseases commonly give rise to suppressed expression of drug-metabolizing enzymes (29, 30). Suppression of CYP expression is primarily due to decreases in the transcription process; but, as a consequence of rising levels of certain inflammatory cytokines, such as IL-1β, IL-6, and TNF-alpha, it may also be due to decreased enzyme activity.
As complications associated with many chronic diseases, infection and inflammation have been proven to affect the hepatic and extra-hepatic metabolism of drugs (31).
In the present study, the effects of type 1 and type 2 diabetes on the activity of the cytochrome 2D1 (the rat orthologue of the human CYP2D6) were compared with that of the control group. The comparison was carried out regarding changes in the metabolic ratio of DXO as the enzyme probe as well as the hepatic clearance status in diabetic rats based on the inlet and outlet concentrations of DXO. Moreover, the effects of the cinnamon administration on the enzyme activity and hepatic clearance, as well as changes in the blood sugar profile, were assessed in both type 1 and 2 diabetic rats. Using the isolated perfused liver model of type 1 and 2 diabetic rats, we examined the effects of insulin injection and metformin administration as routine treatments for type 1 and 2 diabetes mellitus, respectively, on the above-mentioned parameters. This study was designed just to investigate the changes in the CYP2D1 activity by calculating the metabolic ratio of DXO as an accepted probe for these types of studies, independent of the mechanisms of the observed events. Further studies are needed to investigate whether the expression of the enzyme was altered or not.
Based on the average metabolic ratios of the three last sampling time points, the results demonstrated that diabetes mellitus type 1 and 2 significantly decreased the CYP2D1 activity (The The disease also led to decreased hepatic clearance (Figure 3). These alterations in enzyme activity and hepatic clearance were of high importance because they had a modulating effect on liver metabolism and excretion processes for different types of diabetes mellitus.
Based on observations, cinnamon was used as a traditional herbal medicine to lower the blood glucose levels in diabetic patients (32, 33). Thus, cinnamon administration can be considered as an effective way to normalize the CYP2D1 activity (Figure 1) and hepatic clearance (Figure 3) compared with the untreated diabetic rats. Simultaneous administration of cinnamon and insulin has been surprisingly demonstrated to normalize the FBS in the treated type 1 diabetic rats (Figure 4). However, the combined treatment also decreased the CYP2D1 activity compared with the untreated diabetic rats (Figure 1), with no modulating effects on the hepatic clearance in type 1 diabetic rats (Figure 3). This could be attributed to the humanized source of insulin used for the treatment of diabetic rats, probably inducing inflammatory processes in rats. The processes were capable of generating phenoconversion in CYP2D1 activity (30, 34). To prove the hypothesis, it was recommended to evaluate the level of inflammatory cytokines in diabetic rats treated with humanized insulin. On the other hand, simultaneous administration of cinnamon and metformin modulated the CYP2D1 activity (Figure 1) as well as hepatic clearance in type 2 diabetic rats (Figure 3). Thus, the values got close to observed levels in the control group. As indicated, metformin had anti-inflammatory effects (35). Therefore, the effective modulation of enzyme activity observed as the result of using combination therapy in type 2 diabetic rats could be attributed to the blood glucose-lowering (Figure 4) and anti-inflammatory effects of both cinnamon and metformin (32, 33, 35-40). According to the literature review, this is the first study on the benefits of cinnamon consumption on the activity of liver CYP enzymes. Although several studies have approved the hypoglycemic and hypolipidemic effects of different extracts of cinnamon, no similar investigation was found.
4.1. Conclusions
The results of this study indicated that despite suppression of the CYP2D1 activity and reduced hepatic clearance in type 1 and 2 diabetic rats, cinnamon administration could be used as an effective complementary treatment to normalize the metabolism and hepatic clearance processes in diabetes mellitus patients. However, the proposed hypothesis should be evaluated in humans in future research.