1. Background
One of the main public health problems is diabetes mellitus (DM), which affects people at an escalating rate worldwide (1). According to statistics, there is a future estimation of 629 million patients with diabetes by 2045 (2). Hyperglycemia is the main characteristic of diabetes mellitus caused by defects in insulin function. There are two main types of DM: type 1 diabetes (T1D) and type 2 diabetes (T2D). Type 2 diabetes is a chronic metabolic disorder classically identified by hyperglycemia and insulin resistance (3). The overall damaging impact of hyperglycemia are categorized as macrovascular issues (coronary artery disease, peripheral artery disease, and stroke) and microvascular issues (diabetic nephropathy, neuropathy, and retinopathy) (4).
Autoimmune impairment of pancreas β-cells following insulin deficiency leads to insulin resistance (5). One of the factors in the elevation of reactive oxygen species (ROS) is hyperglycemia that causes glycation of scavenging enzymes and reduction in anti-oxidative mechanisms. It is suggested that reduction of ROS production might have an important role in controlling diabetic problems (6). Reactive oxygen species (ROS) are normally erased by various antioxidant defense systems in human bodies. Anti-oxidative enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), as well as non-enzymatic antioxidants, including vitamin C and E function as the antioxidant system in the body fluids (7). Additionally, one of the complications of diabetes is an increase in the incidence of cardiovascular diseases, which causes atherosclerosis through the increase of oxidized LDL and, in most cases, leads to the death of the patient (8). Hyperglycemia caused by uncontrolled diabetes elevates oxidizing agents and LDL oxidation (9). Therefore, in diabetics, lipid profile management, especially cholesterol, could reduce the adverse effects of diabetes on health (10).
Diabetes has different drug treatments depending on its type. Considering the side effects of the drugs discovered in these patients, the correct lifestyle and especially proper nutrition are considered crucial by medical researchers (11). One of the ways to reduce the adverse effects of diabetes, which has been approved by the World Health Organization (WHO), is the use of herbal plants with antidiabetic properties. It has been studied for several years with the aim of reducing the use of chemical drugs and the side effects of chemical drugs in patients with diabetes (12). Research indicated that medicinal plants could ameliorate glucose metabolism in patients with diabetes, boost lipid metabolism, antioxidant mechanism, and capillary function (13). Artemisia dracunculus L. (Tarragon or Estragon), which is famous as “Tarhun” in Anatolia, is a medicinal herb with the main group of biologically vigorous material among plants (12). The antioxidant and antidiabetic effects of this valuable plant have been reported in many animal studies and some clinical studies (14). However, due to the influence of genetics and race on the incidence of diabetes in Iran (15), no study has been conducted regarding the effect of tarragon in diabetic individuals.
2. Objectives
The current interventional clinical trial was designed to assess the wider effects of tarragon oral supplementation on glucose metabolism, insulin resistance, lipid and antioxidant status in patients with T2D during a two-month research.
3. Methods
3.1. Ethics
This research received the approval of the Ethics Committee of Mazandaran University of Medical Sciences, and the registry on Iranian Registry of Clinical Trials website (IRCTID: IRCT20170822035834N3). At the beginning of the study, 70 patients with type 2 diabetes approved by a specialist were included in the study from Endocrine Research Center, Institute of Endocrinology and Metabolism, Mazandaran University of Medical Sciences.
3.2. Inclusion and Exclusion Criteria
This study had the following inclusion criteria: patients with T2D (HbA1c ≥ 6%; or FBS ≥ 126 mg/dL (7 mmol/L); or 2-hp glucose ≥ 200 mg/dL (11.1 mmol/L) or random plasma glucose ≥ 200 mg/dL); tendency to participate in research; ordinary exercise level; stable treatment for at least 2 months. Exclusion criteria were: insulin consumption; sustaining diabetes ≥ 10 years; pregnancy and breastfeeding; body mass index (BMI) > 30; any serious renal and hepatic disorders; any acute condition in cardiovascular, pulmonary, and renal system; malignancies; instability in the dosage of hypoglycemic drugs during diabetes control; any dietary supplement consumption 2 months prior to starting until the end of the research; any allergies; alcohol consumption; smoking; any undesirable effect of the intervention in research; and reluctance to cooperate.
3.3. Patients
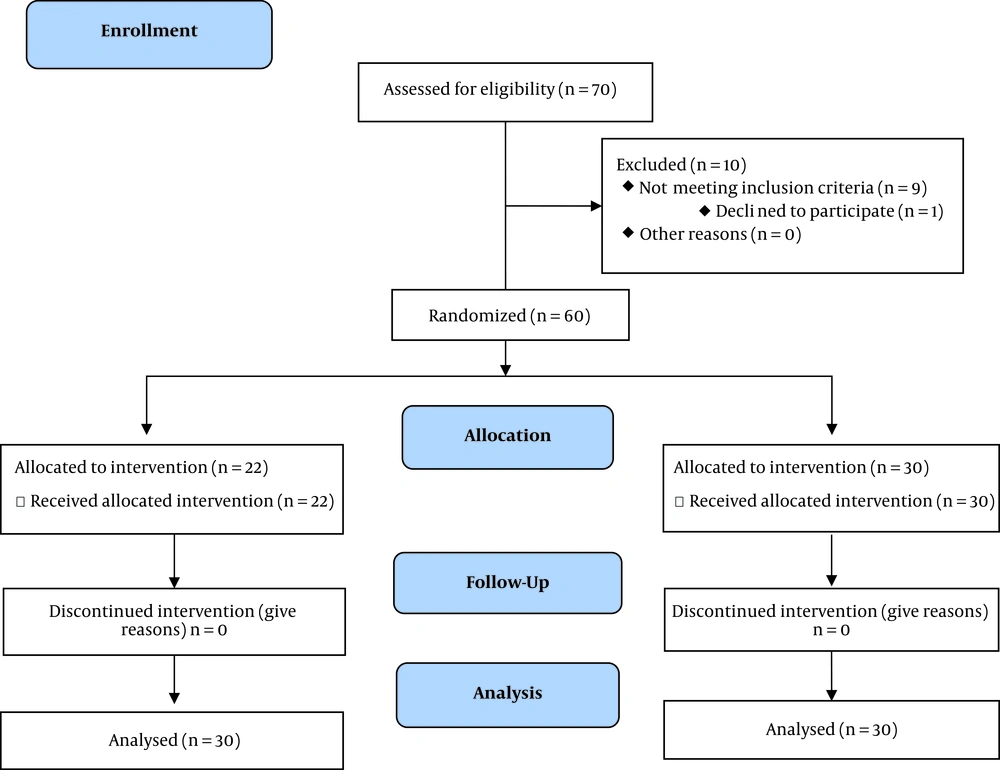
Of 70 diabetics, six patients refused to continue cooperation with the study, and finally, 64 patients entered the study (32 in each group). At the end of the study, 60 patients completely performed the investigation, and four patients quit the research due to the dosage alterations of their treatment. Finally, all sixty patients accepted to willingly participate in this research by their written consent prior to any action.
3.4. Study Design
After justifying the individuals, the demographic characteristics of participants were registered. Then height, weight, and BMI were recorded and matched for age, sex, and weight. A simple random sampling system was used in this research using random numbers. According to this procedure, the patients were categorized into two groups by randomized block based on BMI. In this study, all of the patients, researchers, and specialist physicians were blinded to the intervention supplement and placebo. The patients were randomly allocated to one of the two groups: In the intervention group, patients (n = 30) received three capsules/day, each containing 500 mg tarragon powder for 8 weeks, and the same dose of placebo was administered to the patients of the placebo group (n = 30). The placebo capsules were the same as tarragon capsules in appearance. Patients were in a matched age range and BMI, and were guided to avoid altering their regular diet or physical activity levels during the study. Anthropometric indices, including height and weight, were measured at the beginning and end of the investigation. Moreover, BMI was measured as weight in kilograms divided by the square of height in meters (16). It should be noted that the selection of the prescribed dose in the present study was based on a pilot study performed on ten people and based on the effective dose. Figure 1 shows a description of the study.
3.5. Laboratory Methods
Blood samples were taken after overnight fasting at the start of the study and after two months of intervention as well. After centrifugation of the blood samples, the detached serum was kept at -80°C for further measurements. The levels of fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and triglyceride (TG) were measured by enzymatic method and using an Abbot Model Aclyon 300 auto-analyzer with Pars-Azmoon kits (Tehran, Iran) on the day of sampling. Friedwald equation was used to determine low-density lipoprotein-cholesterol (LDL-C) level calculation (17). An automated high-performance liquid chromatography analyzer with a kit of Bio-Rad D-10 Laboratories (Schiltigheim, France) was used for glycated hemoglobin (HbA1c) measurement. Serum level of insulin was determined by chemiluminescent immunoassay (CLIA) method [LIAISON analyzer (310360) Diasorin S.P.A (Vercelli, Italy). Homeostasis model assessment-insulin resistance (HOMA-IR) was determined by the following formula: Fasting glucose (mg/dL) × fasting insulin (µU/mL)/405 (8).
Total antioxidant capacity (TAC) was determined by a spectrophotometric method using Randox TAS (Laboratories, Crumlin, UK) by an autoanalyzer (Abbott, Model Alcyon 300, USA). In addition, glutathione peroxidase (GPx) and superoxide dismutase (SOD) were measured by spectrophotometric and Randox lab, UK, respectively.
3.6. Sample Size
The serum level of FBS in patients determined the sample size before and after the administration of cinnamon in the study of Khan et al. (18) using the following formula:
Considering α = 0.05 and test power of 90% of the sample size was 19.18 in each group. Considering the 35% probable drop, the sample size was 30 people. In this study, 30 Patients with diabetes (male and female) were included in each group.
3.7. Statistical Analyses
Statistical analyses were performed using SPSS version 20. Results were presented as mean ± SD, and the normality of data distribution was assessed by the Kolmogorov-Smirnov test. To compare the mean biochemical variables before and after the intervention in each group paired sample t-test statistical method was used, and for comparison of variables between two groups independent sample t-test method was used. Differences were considered statistically significant at P < 0.05. In this study, intention-to-treat analysis was not used.
4. Results
The participants with type 2 diabetes (n = 60, 30 in the tarragon group and 30 in the placebo group) completed the study. The characteristics of patients at the start point of the research are presented in Table 1. Based on the independent sample t-test, there was no significant difference in the mean age, weight, BMI, dosages of medication, and duration of diabetes between groups.
| Feature | Tarragon (N = 30) | Placebo (N = 30) | P-Value |
|---|---|---|---|
| Age, y | 54.36 ± 4.27 | 51.07 ± 5.5 | 0.47 |
| Sex | 0.691b | ||
| Male | 17 (56.66) | 16 (53.33) | |
| Female | 13 (43.33) | 14 (46.66) | |
| Weight, kg | |||
| Initial | 70.19 ± 10.2 | 71.09 ± 8.45 | 0.71 |
| End | 69.05 ± 9.13 | 70.73 ± 8.07 | 0.74 |
| BMI, kg/m2 | |||
| Initial | 26.42 ± 4.1 | 27.13 ± 4.25 | 0.75 |
| End | 26.05 ± 3.14 | 26.99 ± 3.83 | 0.84 |
| Metformin dose, mg | 1523.37 ± 394.1 | 1513.17 ± 409.04 | 0.88 |
| Diabetes duration, y | 6.04 ± 2.13 | 6.51 ± 2.03 | 0.81 |
aValues are expressed as No. (%) or mean ± SD.
bComparison of mean baseline features by independent samples t-test.
The mean glucose index of the patients is shown in Table 2. According to the independent sample t-test, there was no statistically significant difference in total FBG, 2-hour postprandial (2hpp), insulin, HOMA-IR, and HbA1c% between two groups before the treatment (P > 0.05, Table 2). Tarragon supplementation reduced FBG, 2-hpp, insulin, HOMA-IR, and HbA1c% (P < 0.05) compared to the placebo receivers. Based on paired sample t-test, it was indicated that the FBS, 2-hp, insulin, HOMA-IR, and HbA1c% significantly decreased after the intervention in the Tarragon treated group (P < 0.05).
| Variables | Tarragon (N = 30) | Placebo (N = 30) | P1 |
|---|---|---|---|
| FBS, mg/dL | |||
| Baseline | 163.1 ± 19 | 159.07 ± 21.06 | 0.74 |
| End | 123.07 ± 19.3 | 160.11 ± 31.15 | 0.021 |
| P2 | 0.019 | 0.872 | |
| 2-hp, mg/dL | |||
| Baseline | 196.02 ± 73.11 | 199.13 ± 80.19 | 0.66 |
| End | 159.14 ± 61.2 | 199.27 ± 78.3 | 0.037 |
| P2 | 0.04 | 0.82 | |
| Insulin, µU/mL | |||
| Baseline | 12.07 ± 3.42 | 12.39 ± 3.09 | 0.301 |
| End | 10.01 ± 2.17 | 12.69 ± 3.4 | 0.01 |
| P2 | 0.024 | 0.56 | |
| HOMA-IR | |||
| Baseline | 4.86 ± 0.16 | 4.84 ± 0.21 | 0.9 |
| End | 3.04 ± 0.1 | 5.01 ± 0.26 | 0.017 |
| P2 | 0.034 | 0.19 | |
| HbA1c, % | |||
| Baseline | 8.07 ± 1.3 | 7.9 ± 1.41 | 0.42 |
| End | 6.6 ± 1.02 | 7.85 ± 1.39 | 0.029 |
| P2 | 0.033 | 0.7 |
Abbreviations: FBS, fasting blood sugar; HbA1c (%), hemoglobin A1c (%); HOMA-IR, homeostasis model assessment of insulin resistance; SD, standard deviation; 2-hp, two-hour postprandial glucose.
aValues are expressed as No. (%) or mean ± SD.
bP < 0.05, compared with the control group.
cP1, Comparing the mean of variables between the two groups (the statistical analyzes independent samples t-test); P2, comparing the mean of variables in each group at the baseline and end of the study (the statistical analysis paired samples t-test).
The effect of tarragon on lipid profile is presented in table 3. Based on the independent sample t-test, there was no significant difference in lipid profile among participants of two groups at the start of the study. The mean values of TC, TG, and LDL in the tarragon group significantly reduced after eight weeks (P < 0.05). Changes in HDL levels were not significant (P = 0.12) (Table 3).
| Variables | Tarragon (N = 30) | Placebo (N = 30) | P1 |
|---|---|---|---|
| TC, mg/dL | |||
| Baseline | 252.01 ± 50.37 | 242.11 ± 46.22 | 0.501 |
| End | 184.5 ± 45.13 | 239.07 ± 50.01 | 0.036 |
| P2 | 0.001 | 0.711 | |
| TG, mg/dL | |||
| Baseline | 239.31 ± 67.01 | 248.13 ± 65.21 | 0.671 |
| End | 209.02 ± 49.14 | 242.41 ± 64.05 | 0.042 |
| P2 | 0.035 | 0.7 | |
| HDL-c, mg/dL | |||
| Baseline | 42.07 ± 4.36 | 41.9 ± 3.08 | 0.4 |
| End | 44.21 ± 4.09 | 42.11 ± 5.1 | 0.43 |
| P2 | 0.12 | 0.37 | |
| LDL-c, mg/dL | |||
| Baseline | 129.94 ± 33.01 | 149.11 ± 30.14 | 0.061 |
| End | 98.29 ± 31.02 | 148.47 ± 32.1 | 0.023 |
| P2 | 0.042 | 0.83 |
Abbreviations: HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; SD, standard deviation; TC, Total cholesterol; TG, triglyceride.
aValues are expressed as No. (%) or mean ± SD.
bP < 0.05, compared with the control group.
cP1, Comparing the mean of parameters between the two groups (the statistical analyzes independent samples t-test); P2, comparing the mean of parameters in each group at the baseline and end of the study (the statistical analysis paired samples t-test).
The mean value of anti-oxidative parameters is shown in Table 4. In the tarragon group, the mean values of TAC, SOD, and GPx increased (P < 0.05) that was statistically significant. However, there was no significant change in anti-oxidative values of the placebo group (P > 0.05).
| Variables | Tarragon (N = 30) | Placebo (N = 30) | P1 |
|---|---|---|---|
| TAC, mg/dL | |||
| Baseline | 0.89 ± 0.17 | 0.98 ± 0.71 | 0.518 |
| End | 1.34 ± 0.27 | 0.83 ± 0.22 | 0.039 |
| P2 | 0.021 | 0.491 | |
| SOD, µU/mL | |||
| Baseline | 1611.03 ± 219.23 | 1647.35 ± 254.01 | 0.604 |
| End | 1841 ± 309.4 | 1621.61 ± 229.3 | 0.027 |
| P2 | 0.02 | 0.564 | |
| GSH-Px, mg/dL | |||
| Baseline | 40.73 ± 8.61 | 38.11 ± 7.39 | 0.601 |
| End | 47.49 ± 9.27 | 37.63 ± 8.09 | 0.027 |
| P2 | 0.033 | 0.404 |
Abbreviations: GSH-Px, glutathione peroxidase; SD, standard deviation; SOD, Super oxide dismutase; TAC, total antioxidant capacity.
aValues are expressed as No. (%) or mean ± SD.
bP < 0.05, compared with the control group.
cP1, Comparing the mean of variables between the two groups (the statistical analyzes independent samples t-test); P2, comparing the mean of variables in each group at the baseline and end of the study (the statistical analysis paired samples t-test).
5. Discussion
Despite the presence of noted antidiabetic medicines, herbal productions are vastly appreciated by researchers and are detected to be less harmful with less unfavorable effects than synthetic drugs (4). Research has detected that the ingredients of tarragon show powerful anti-oxidative activities (19). The current study indicates that 1.5 g/d tarragon supplementation for 8 weeks significantly improves FBG levels and lipid profiles in patients with type 2 diabetes.
The result of in vitro and in vivo investigations proves the role of tarragon in glycemic control. Studies of tarragon extracts indicate an increased dose-dependent anti-hyperglycemic effect. Tarragon bioactive ingredients serve as a useful remedy for diabetes in Streptozotocin-induced diabetic rats, showing hyperglycemia and hyperlipidemia (20). Ghazanfar et al. (19) studied antidiabetic potential of tarragon. According to their results, tarragon could improve hyperglycemia and protect rats against other metabolic problems caused by diabetes. Their results showed that hydroethanolic extract of tarragon significantly decreased glucose levels in diabetic rats. Moreover, the histopathological results of their treated groups proved the regenerative/protective effect of tarragon on pancreas β-cells in diabetic rats (19). Furthermore, Ahmad et al. (14) showed that a daily oral consumption of hydromethanolic crude extracts (200 and 400 mg/kg b.w.) and chloroform fraction of tarragon for 15 days decreased blood glucose level, which were similar to the standard antidiabetic agent, glibenclamide. Extracts of tarragon improved glycemic status in oral glucose tolerance tests. The results showed that tarragon extracts reduced blood glucose levels in normal rats as well. This phenomenon could result from the increased effectiveness of the peripheral tissues to uptake blood glucose (14).
Govorko et al. (11) evaluated the polyphenolic compounds of tarragon that inhibit phosphoenolpyruvate carboxykinase (PEPCK) gene expression and gluconeogenesis in an H4IIE hepatoma cell line. They observed that two polyphenolic compounds increased PEPCK expression at mRNA levels (11). Tarragon exerts its effect on reducing insulin resistance by activating the phosphoinositide-3 kinase (PI3K) pathway. It has been revealed that tarragon has an interaction with PI3K (21). Hepatic gluconeogenesis is regulated by several factors, and PEPCK has an essential role in hepatic glucose output. As the expression level of the PEPCK gene increases, the expression of glucocorticoids and glucagon mounts, which leads to an increase in blood glucose levels. This up-regulation happens through cyclic adenosine monophosphate (cAMP) (22). On the contrary, the expression of the PEPCK gene decreases when circulating blood insulin increases because of postprandial hyperglycemia. In type 2 diabetic insulin resistance, the elevated expression of PEPCK due to glucagon action causes the reduction of insulin response in the liver. This allows ongoing hepatic glucose yield, significant hyperglycemia, and diabetes-related complications. Some present drugs, such as metformin and thiazolidinedione, are capable of suppressing basal PEPCK expression but not hormone-caused PEPCK induction. As a result, these drugs lack the essential potency to boost insulin sensitivity (23).
Elevated plasma levels of TC, TG, and LDL-C are the crucial risk factors of cardiovascular disorders (24). The results of the current study show that tarragon powder leads to significant improvements in the lipid profile of individuals with type 2 diabetes, which is consistent with the study of Roghani et al. (cited in Parsaei et al.), who established the efficacy of tarragon powder in reducing TG levels in diabetic rats (25).
Many scientific reports demonstrate the correlation of oxidative stress with several pathological conditions involving DM (26). According to the clinical studies, the concentration of SOD, GPx, TAC, and other antioxidants such as vitamin E decrease in patients with uncontrolled diabetes. SOD is particularly disrupted before other anti-oxidative enzymes because it is regarded as the first-line defense system against oxidative stress (10). Jarouliya et al. (27) demonstrated that Spirulina improved blood glucose levels and reduced oxidative markers due to its anti-oxidative properties. Our findings indicate that supplementation with 1500 mg/day of tarragon leads to significant improvements in blood antioxidant parameters of individuals with type 2 diabetes; TAC, GPx, and SOD increased significantly after adjuvant therapy with tarragon.
Animal research indicates that tarragon extracts might induce regeneration/proliferation of the pancreatic β-cells through the inhibition of free radical formation (19). Neogenesis or replication of the pre-subsisting differentiated cells form the new pancreatic β-cells, and tarragon extract potentiates this process (28). Also, mitogen-activated protein kinases (MAPK) signaling cascade is triggered by ROS generation in the human body (29). Sadek et al. (30) proved that Spirulina has a significant positive impact on MAPK activity.
The current clinical research, like many studies, has strong and weak points. One strong point is that this research is the pioneer to investigate the effect of the tarragon powder supplement on Iranian individuals with diabetes on glycemic and antioxidant status. Moreover, the design of this study as a double-blind, randomized clinical trial that had parallel groups causes the results of this research to be extraordinary. However, complications were the limited budget, and number of participants, and the period of the medical intervention. In order to achieve more concise and strong conclusions, it is required to conduct further researches with a larger population group and intervention period.
5.1. Conclusions
This study established that eight weeks of supplementation with tarragon powder decreased the levels of blood sugar, blood lipids, and IR in patients with diabetes. Based on these results, there is evidence supporting the view that polyphenol antioxidant groups can reduce the complications of diabetes and play a key role in the management of diabetic conditions. Nevertheless, further studies are needed to provide additional evidence in a vast study group.