1. Background
Inflammation is an immune response against the invasion of foreign objects, tissue damage, or both (1, 2). The causes of inflammation include mechanical trauma, microorganism, physical effects, and chemical substances (3). The symptoms of anti-inflammatory response consist of swelling (tumor), heat (calor), pain (dolor), and redness (rubor) that inflammatory mediators such as prostaglandin, histamine, leukotriene, and serotonin, are being released (3, 4). Meanwhile, anti-inflammatory agents are defined as a medicine that is capable of suppressing or inhibiting inflammation, where their working mechanism, either the steroid or non-steroid group, is through the release of prostaglandin toward the injured tissue (5, 6)
The administration of anti-inflammatory agents by topical procedure, generally, is better than the oral administration, because it does not go through the first pass effect and digestive tract (7, 8). The topical administration of anti-inflammatory agents only gives a local effect on the injured part, thus reducing the side effects (7). Nevertheless, the systemic effects of the topical administered medicine depend on the skin penetration ability to enter the circulation or absorb into the deeper tissue to inhibit cyclooxygenase activity (9, 10). There are many preparations available for topical administration, such as cream, ointment, and gel. Gel preparations are more preferable due to their good distribution on the skin, cooling effect, absence of physiological inhibition against the hair function, washable property, and good medicine (active compounds) release (8, 9, 11). The active compounds used in this study is the nanoparticle from arabica coffee grounds (Coffea arabica L.) owing to having active compounds such as phenolics (chlorogenate acid (12) and flavonoid (13) with total phenolics 1246.90 μgGAE/g (14), alkaloids (15), carotenoids, saponins (16, 17) and others (18, 19). In addition, the arabica coffee grounds contain essential oil (20). Those compounds are reported to have antioxidant (21) and anti-inflammatory effects (22-26).
This study is also a continuation of our previous work; Nurman et al. (2019) (27), about optimizing the gelling of nanoparticles arabica coffee grounds. The results of this study suggest that the arabica coffee ground nanoparticles gel preparations have good physicochemical properties with a pH value of 5.212, 3734.244 cps viscosity, 5.850 cm spread power, and 669.277 µgGAE/g total phenolic content. After obtaining a food gel, thus in this study, we proceed with the investigation of the anti-inflammatory activities on carrageenan-induced male mice. The optimization of the inflammatory inhibition of arabica coffee grounds gel done with the Box-Behnken design model of the response surface methodology on Design Expert Version 10.0.3.0 software (28, 29), employing three levels of three factors, including Carbopol 940, triethanolamine, and arabica coffee ground nanoparticles.
2. Objectives
The tools used in this study included the laboratory equipment, such as volumetric glass, analytical balance, beaker glass, got plate, magnetic stirrer, volumetric pipette, pestle, mortal, and syringe. Meanwhile, the instrument used is digital plethysmometer (TM Orchid Scientific, Orchid Scientific & Innovative India Pvt Ltd, Maharashtra, India). The ingredients in this study are Carbopol 940, carrageenan, glycerin, NaCl, methylparaben, and triethanolamine (TEA) (Pharmaceutical Grade), Arabica coffee grounds (Coffea arabica L.) nanoparticles, and distilled water. Voltaren gel (with natrium diclofenac) (PT. Novartis Indonesiai) was used as the positive control, and the negative control was base gel (gel without nanoparticle), as well as male mice (Mus musculus) were used as the experimental animal from strain Balb/c, aged 2-3 months and with 20 - 30 g body weight.
3. Methods
3.1. Ethical Approval
This study was approved by the Ethics Committee of Health Research, Medical School, Syiah Kuala University, District Public Hospital dr. Zeinoel Abidin Banda Aceh, Aceh Province, Indonesia No. 320/EA/FK-RSUDZA/2019.
3.2. Formulation of Arabica Coffee Ground Nanoparticles Gel
Carbopol 940 with the variation of 0.5; 0.75 to 1.00% was respectively dissolved in 100 mL distilled water, heated to 80°C for 30 min, and stirred with a magnetic stirrer. After the Carbopol had expanded, it was placed in the mortar, TEA was added with the variation of 0.40; 0.50 to 0.60%, and 7.5 mL glycerin; it was ground until it reached a homogeneity. Afterward, 0.1 g methylparaben was added and stirred constantly; subsequently, nanoparticles of arabica coffee grounds were added with a variation of 1.50; 2.25 to 3.00%, followed by another grinding until it turned homogenous (27). Variations of Carbopol 940, TEA, and nanoparticles of arabica coffee grounds were chosen because these materials could affect the viscosity, pH, and anti-inflammatory properties of the gel produced.
3.3. Preparation of the Experimental Animals
The experimental animals used were male mice (Mus musculus) from strain Balb/c, aged 2 - 3 months, with 20 - 30 g body weight. All 20 mice were acclimatized for one week through feed and water intake. After one week, the mice were fasted for 8 h before the experiment.
3.4. Anti-inflammatory Activities Test
During the test, each mice was cleaned, and its left foot was marked. Then, the marked foot was measured with plethysmometer and noted as initial volume (Vo); the foot volume before the intervention. The foot sole was given an interplanar injection of 0.1 mL carrageenan suspension 1%. The foot volume was re-measured 30 min after the injection. Next, each group was given topical intervention according to the treatment group (arabica coffee ground nanoparticles gel-treated test group, positive control group, and negative control group). After 60 min, the marked foot was re-measured as t-time volume (Vt). The measurement of the mice’s foot was conducted until 360th min with a 30 min time difference (1).
3.5. Data Analysis
Edema volume was calculated from the mice’s foot measurement using Equation 1, the Area Under Curve (AUC) was calculated using Equation 2, and inflammatory inhibition was calculated using Equation 3 (3). The optimization of anti-inflammatory activities of arabica coffee ground nanoparticles gel was assessed using Box-Behnken design of the response surface methodology in Design Expert Version 10.0.3.0 software as follows:
I. Generating the formulation design and response based on the experimental design.
II. Formulation step; carrying out the experiment according to the determined formula condition.
III. Analyzing the resulted response.
IV. Conducting an optimization, followed by verification as the evidence against the predicted response value of optimum formula solution.
Information:
Vu: Edema foot volume
Vt: t-time foot volume
Vo: Initial foot volume
Vun-1: Average edema volume at tn-1-time
Vun: Average edema volume at tn-time
AUCk: AUC of edema volume curve for negative control group
AUCp: AUC of edema volume curve for test group
4. Results and Discussion
4.1. Design Formulation of Coffee Grounds Nanoparticle Gel
Box-Behnken Design on Design Expert Version 10.0.3.0 software used to obtain a combination of gel formulation treatment with variations of Carbopol 940 (0.5; 0.75 and 1.00 %), TEA (0.40; 0.50 and 0.60 %) and arabica coffee ground nanoparticles (1.50; 2.25 and 3.00 %), resulting in the design as shown in Table 1 (27).
| Run | Factor 1, A: Carbopol 940 (%) | Factor 2, B: TEA (%) | Factor 3, C: Nanoparticles (%) |
|---|---|---|---|
| 1 | 1.00 | 0.50 | 1.50 |
| 2 | 0.75 | 0.50 | 2.25 |
| 3 | 1.00 | 0.40 | 2.25 |
| 4 | 0.50 | 0.40 | 2.25 |
| 5 | 0.50 | 0.50 | 3.00 |
| 6 | 0.75 | 0.60 | 1.50 |
| 7 | 0.75 | 0.40 | 1.50 |
| 8 | 0.75 | 0.50 | 2.25 |
| 9 | 0.75 | 0.50 | 2.25 |
| 10 | 0.75 | 0.50 | 2.25 |
| 11 | 0.75 | 0.40 | 3.00 |
| 12 | 0.75 | 0.60 | 3.00 |
| 13 | 0.50 | 0.50 | 1.50 |
| 14 | 0.75 | 0.50 | 2.25 |
| 15 | 1.00 | 0.50 | 3.00 |
| 16 | 0.50 | 0.60 | 2.25 |
| 17 | 1.00 | 0.60 | 2.25 |
The design model selected in this research is quadratic model owing to the high score of R-square and low score of Prediction Residuals Error Sum of Square (PRESS), as seen in Table 2. Pred R-Squared value of 0.7390 and Adj R-Squared value of 0.7758 with a difference of less than 0.2. The expected ratio is higher than 4 and the ratio of 7.647 can produce an adequate signal (29, 30). This design model can be used to control the design space.
| Source | Std.Dev | R-Square | Adj R-Square | Pred R-Square | Adeq Precisior | PRESS |
|---|---|---|---|---|---|---|
| Linear | 1.56 | 0.1329 | -0.0672 | -0.5059 | 2.381 | 55.28 |
| 2FI | 1.73 | 0.1891 | -0.2974 | -1.8812 | 2.326 | 105.77 |
| Quadratic | 0.72 | 0.9019 | 0.7758 | 0.7390 | 7.647 | 9.58 |
| Cubic | 0.91 | 0.9094 | 0.6375 | - | 5.269 | - |
4.2. Anti-inflammatory Activities
The anti-inflammatory activities or inflammatory inhibition of arabica coffee ground nanoparticles gel are determined by measuring the edema volume of the carrageenan-induced mice foot. The increase of edema volume of the mice’s foot from the first to 180th min was caused by the release of inflammation mediator such as histamine, prostaglandins, bradykinins, and serotonin (31, 32). Meanwhile, at the 240th min, the edema of the mice’s foot experienced a decrease due to the inhibition of prostaglandins synthesis (1, 5). The measurement of the mice’s foot edema in this study exhibits the average inflammatory inhibition values of 27.75%, 27.11%, 0.00% for arabica coffee ground nanoparticles gel (Run 1-17), nanoparticle powder, and negative control, respectively (see Table 3). It suggests that the inflammatory inhibition of arabica coffee ground nanoparticles gel has ½ times ability of the positive control.
| Run | Edema Volume | Total AUC | Inflammatory Inhibition (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 60' | 120' | 180' | 240' | 300' | 360' | |||
| 1 | 0.133 | 0.190 | 0.234 | 0.183 | 0.148 | 0.075 | 1.931 | 25.12 |
| 2 | 0.128 | 0.183 | 0.224 | 0.172 | 0.134 | 0.017 | 1.797 | 30.34 |
| 3 | 0.130 | 0.187 | 0.229 | 0.176 | 0.140 | 0.025 | 1.849 | 28.30 |
| 4 | 0.121 | 0.177 | 0.235 | 0.180 | 0.130 | 0.018 | 1.828 | 29.12 |
| 5 | 0.133 | 0.203 | 0.228 | 0.177 | 0.149 | 0.057 | 1.891 | 26.67 |
| 6 | 0.160 | 0.183 | 0.227 | 0.185 | 0.162 | 0.030 | 1.921 | 25.53 |
| 7 | 0.134 | 0.191 | 0.236 | 0.183 | 0.158 | 0.008 | 1.913 | 25.82 |
| 8 | 0.134 | 0.190 | 0.233 | 0.169 | 0.135 | 0.022 | 1.857 | 28.01 |
| 9 | 0.126 | 0.186 | 0.231 | 0.185 | 0.125 | 0.048 | 1.826 | 29.18 |
| 10 | 0.135 | 0.180 | 0.237 | 0.169 | 0.136 | 0.068 | 1.847 | 28.37 |
| 11 | 0.131 | 0.188 | 0.232 | 0.178 | 0.142 | 0.021 | 1.863 | 27.75 |
| 12 | 0.134 | 0.189 | 0.233 | 0.189 | 0.133 | 0.033 | 1.888 | 26.79 |
| 13 | 0.134 | 0.194 | 0.236 | 0.182 | 0.146 | 0.007 | 1.903 | 26.23 |
| 14 | 0.133 | 0.191 | 0.235 | 0.169 | 0.128 | 0.039 | 1.822 | 29.36 |
| 15 | 0.126 | 0.185 | 0.230 | 0.179 | 0.138 | 0.084 | 1.866 | 27.63 |
| 16 | 0.122 | 0.188 | 0.239 | 0.190 | 0.134 | 0.017 | 1.850 | 28.26 |
| 17 | 0.132 | 0.187 | 0.229 | 0.176 | 0.139 | 0.026 | 1.823 | 29.32 |
| Nanoparticles | 0.131 | 0.185 | 0.229 | 0.178 | 0.145 | 0.052 | 1.880 | 27.11 |
| Negative control | 0.136 | 0.190 | 0.262 | 0.266 | 0.272 | 0.273 | 2.579 | 0.0 |
| Positive control | 0.125 | 0.159 | 0.146 | 0.107 | 0.063 | 0.010 | 1.283 | 50.24 |
Table 4 represents that the quadratic model is significant as shown by the F-value of 7.15 and the value of "Prob> F" with a value of less than 0.0500. The nanoparticle factor (C and C2) has a value of less than 0.0500, so the factor is significant. "Lack of Fit F-value" has a value of 0.11, which means it is not significant relative to pure error. There is a 95% chance that a "Lack of Fit F-value" of this magnitude can occur due to noise. There is a relationship between inflammatory gel inflammation with a factor (x) of the coefficient values, as seen in the Equation 4 (1).
| Source | Sum Of Squares | Df | Mean Square | F-Value | P-Value, Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 33.11 | 9 | 3.68 | 7.15 | 0.0084 | Significant |
| A-carbopol 940 | 8.292 × 10-4 | 1 | 8.292 × 10-4 | 1.612 × 10-3 | 0.9691 | |
| B-TEA | 0.14 | 1 | 0.14 | 0.28 | 0.6127 | |
| C-nanoparticles | 4.73 | 1 | 4.73 | 9.20 | 0.0190 | |
| AB | 0.88 | 1 | 0.88 | 1.70 | 0.2330 | |
| AC | 1.08 | 1 | 1.08 | 2.09 | 0.1915 | |
| BC | 0.11 | 1 | 0.11 | 0.21 | 0.6573 | |
| A2 | 0.14 | 1 | 0.14 | 0.26 | 0.6235 | |
| B2 | 0.063 | 1 | 0.063 | 0.12 | 0.7365 | |
| C2 | 25.46 | 1 | 25.46 | 49.49 | 0.0002 | |
| Residual | 3.60 | 7 | 0.51 | |||
| Lack of fit | 0.27 | 3 | 0.091 | 0.11 | 0.9500 | Not significant |
| Pure error | 3.33 | 4 | 0.83 | |||
| Cor total | 36.71 | 16 |
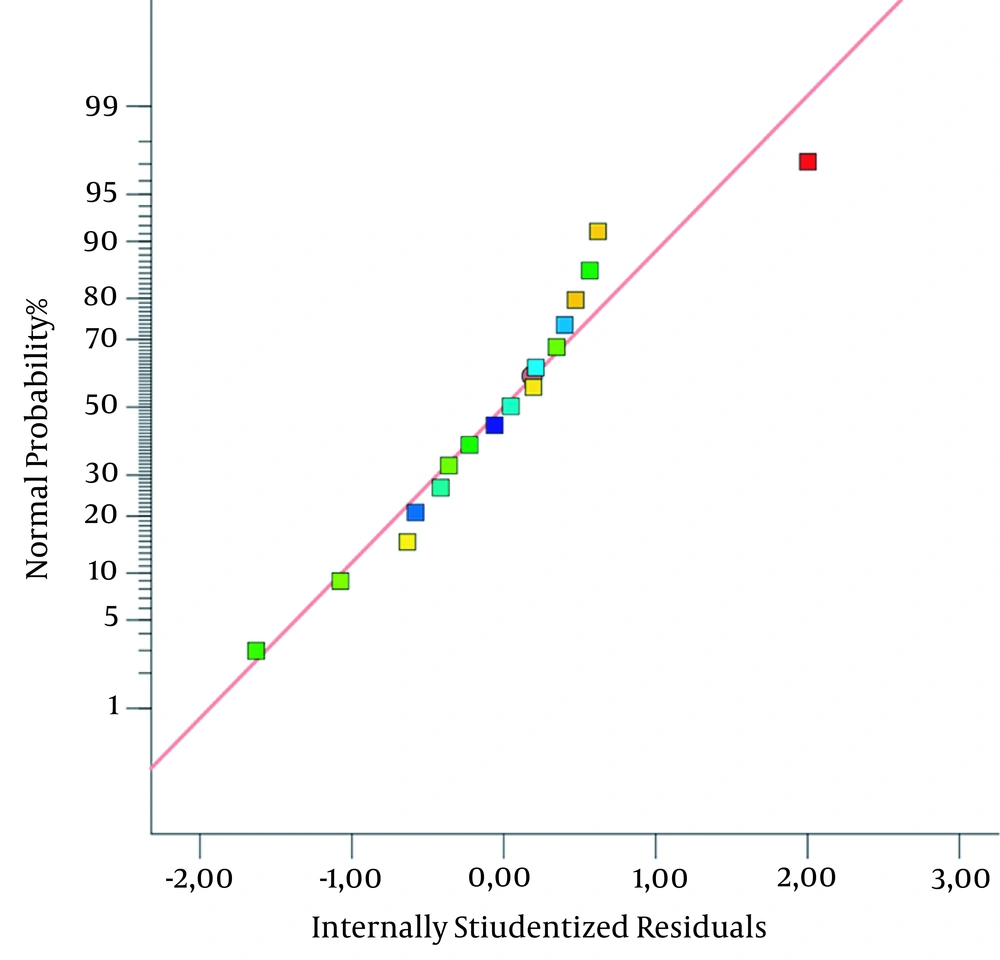
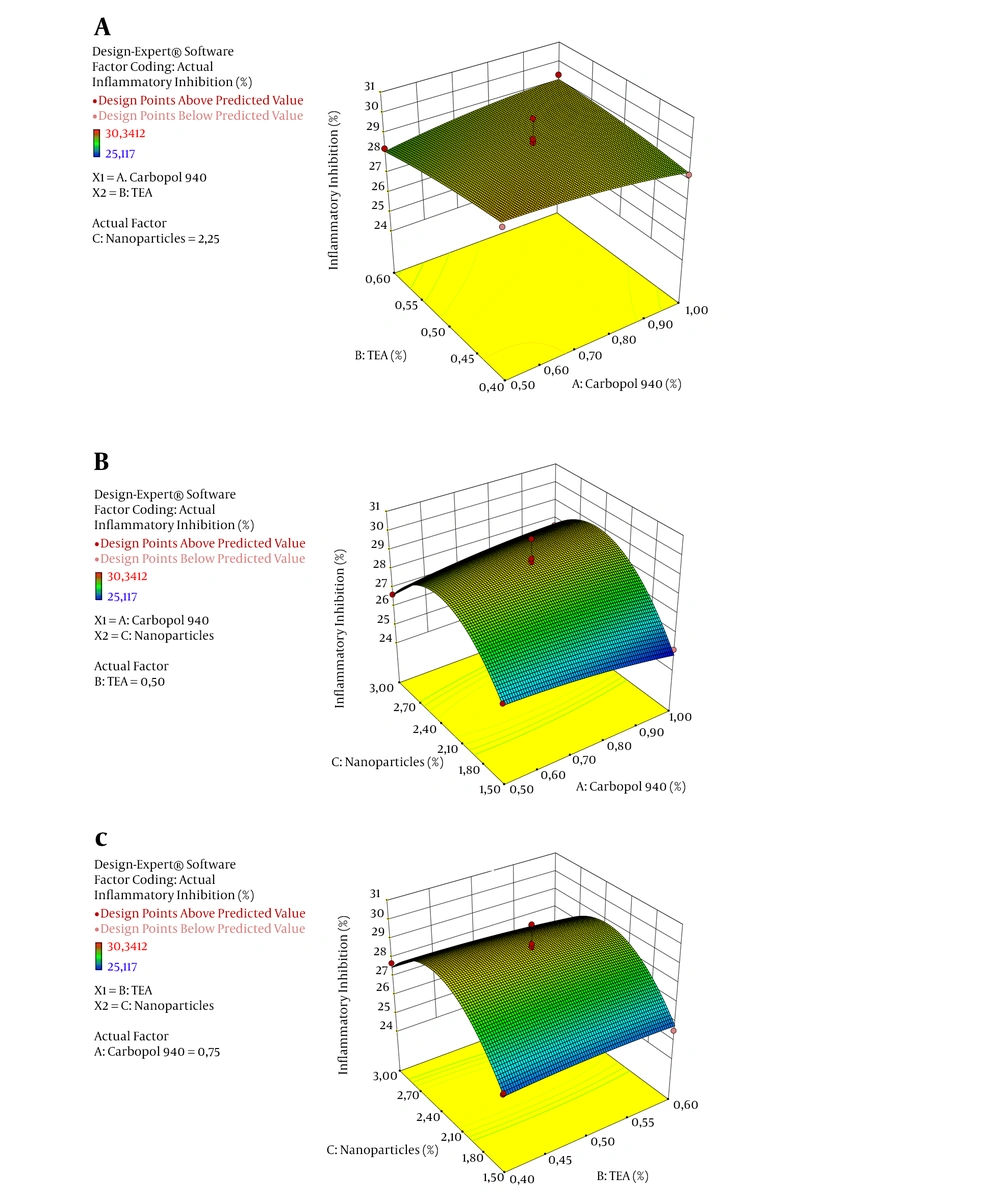
Figure 1 shows the graph of the normal plot of residuals, which can be used to identify the correlation of actual and predicted values. The picture shows that inflammatory inhibition values are close to the normality line, suggesting a normal distribution of the data. It further suggests, the actual result will be found to be close to the program-predicted values. The 3D plot of the correlation of inflammatory inhibition factors, as shown in Figure 2. Moreover, a significant effect of arabica coffee ground nanoparticles is seen on the inflammatory inhibition, according to the curved graph. However, Carbopol 940 and TEA did not affect the inflammatory inhibition, according to the flat-shaped graph. It is corroborated by the inflammatory inhibition value of the negative control, which is the base of the preparations gel.
4.3. Optimization of Arabica Coffee Ground Nanoparticles
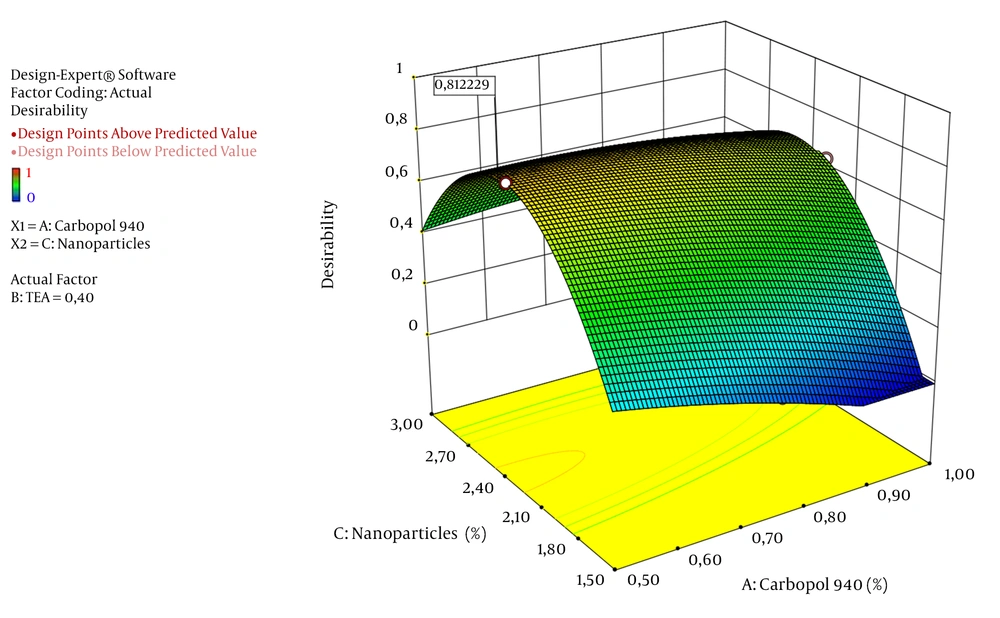
General combination of the formulation of the arabica coffee ground nanoparticles gel using Box-Behnken Design of Design Expert Version 10.0.3.0 software is 0.500% Carbopol 940, 0.400% TEA, and 2.313% nanoparticles, giving 29.360% and 29.670% predicted and experimental inhibition inflammatory values, respectively. Meanwhile, the desirability value is 0.812, which is seen in Table 5. The 3D plot graph of the desirability can be seen in Figure 3, where the desirability value is observable at point 0.8122229.
| Carbopol 940 | TEA | Nanoparticles | Inflammatory Iinhibition – Prediction | Inflammatory Inhibition Experiment | Desirability |
|---|---|---|---|---|---|
| 0.500 | 0.400 | 2.314 | 29.360 | 29.670 | 0.812 |
5. Conclusions
Arabica coffee ground nanoparticles gel can be used as anti-inflammatory medicine with the inflammatory inhabitation degree of the ½ of the commercial medicine (Voltaren gel). This research demonstrates that the inflammatory inhibition optimization of the formulation of arabica coffee ground nanoparticles gel can be conducted by using response surface methodology with Box-Behnken Design using Design Expert Version 10.0.3.0 software. It may be due to the fact that both the predicted and experimental inflammatory inhibition values are relatively similar.