1. Background
Malaria is a well-known parasitic disease and an important public health problem, particularly in tropical and subtropical areas. Despite many efforts, this infectious disease infected over 200 million cases and caused 438000 deaths worldwide in 2015 (1-3). The disease is transmitted by the bite of the Plasmodium-infected female Anopheles sp. mosquito, and it is caused by intracellular parasitic protozoa of the genus Plasmodium (4).
Moreover, the disease is one of the most prevalent parasitic infections in Southeastern Iran (Sistan and Baluchestan, Hormozgan, and the tropical areas of Kerman province), constituting about 95% of all malaria cases in the country (5).
Studies on malaria treatment indicate the necessity of introducing new anti-malaria medications due to increasing rates of drug resistance (4). Nowadays, medicinal plants, as invariable resources for new drugs, have been investigated for their antimalarial properties (6). As an ongoing effort to divulge the antimalarial effects of Iranian plants, we here studied the antimalarial properties of the extracts and fractions with different polarities of Ecbalium elaterium (L.) Rich.
Ecballium elaterium or wild cucumber (family: Cucurbitaceae), which has wide-range traditional usages, is a monotypic weedy species growing by the roadside and waste grounds from Northern Spain through Southern Europe to the Mediterranean region, Southwest Asia, and North Africa (7, 8). It also grows as a perennial herbaceous plant in Gilan and Azerbaijan provinces of Iran (9). In folk medicine, different parts of the plant are used for treating rhinosinusitis, sinusitis, malaria, psoriasis, rheumatism, constipation, jaundice, otitis, hepatitis, hemorrhoid, epilepsy, and chronic headache and as an emetic and emollient agent (10-15).
Previous reports revealed several pharmaceutical and biological effects for E. elaterium, such as antimicrobial (16), anti-inflammatory (17), anti-trypsin (13), anti-insect (18), antioxidant, cytotoxic (19), and hepato-protective (20) activities. On the contrary, there are several studies that introduce E. elaterium as a toxic herb, particularly its juice, with poisonous effects against the respiratory, gastrointestinal, and cardiovascular systems (8).
2. Objectives
The present study aimed to evaluate (a) the antimalarial effects of different extracts of the fruits, seeds, and rhizomes of E. elaterium, (b) fractionize the most potent extract, (c) characterize the most potent parts, and finally (d) identify their structural groups using the Thin-Layer Chromatography (TLC) method.
3. Methods
3.1. Chemicals
The solvents that were used for extraction and fractionation were bought from Duksan Laboratories (South Korea). Chloroquine diphosphate, Hematin porcine, sodium lauryl sulfate (SLS), Glucose, Magnesium sulfate, Sodium ethanoate, Sodium phosphate dibasic, Potassium chloride, Sodium chloride, Caustic soda, and sodium hydrogen carbonate were obtained from Sigma Aldrich Company (the UK). Oleic acid was purchased from Fluka (India); HCl (Hydrochloric acid) and Dimethyl sulfoxide (DMSO) from Merck (Germany); and C18 Sep-Pak cartridge from Waters (the US).
3.2. Plant Materials
Different parts of E. elaterium were collected during July and August 2015 from the Moghan region in Ardabil province, Iran, which has an altitude of 191 m above sea level (48° 20’ 75’’ N, 39° 38’ 63’’ E). A voucher sample (Tbz-FPh.648) was consigned to the Faculty of Pharmacy herbarium, Tabriz University of Medical Sciences, Tabriz, Iran.
3.3. Extraction, Separation, and Identification of Compounds
The dried and crushed fruits, seeds, and roots of E. elaterium (150 g) were consecutively extracted by the n-Hex, DCM, and MeOH solvents using the soxhlet system (1000 mL of each solvent).
Considering the significant antimalarial effects of the MeOH root extract, about 2 g of the mentioned extract was fractionalized using a C18 Sep-Pak cartridge and the solid phase extraction (SPE) method, eluting in a MeOH/water mixture gradient (10:90, 20:80, 40:60, 60:40, 80:20, and 100:0). All these nine extracts and six fractions were distinctly concentrated by an evaporator at 45°C.
3.4. Antimalarial Cell-free Assay
The antimalarial activities of the plant extracts and fractions were assessed using the method described by Afshar et al. with some changes (21). Briefly, various concentrations (0.1 – 2 mg/mL in DMSO) of the samples were incubated with hematin (3 mM, 100 µL), oleic acid (10.0 mM, 10 µL), and HCl (1 M, 10 µL). The last volume was added to 1 mL sodium acetate buffer (pH = 5) and incubated overnight at 37°C with continuous mild shaking. Afterward, the samples were centrifuged, and the hemozoin pellet was adequately washed and incubated with 2.5% SDS in phosphate buffer at 37°C (15 min) under regular shaking. In the next step, the samples were washed in sodium bicarbonate (0.1 M, 1 mL) until a clear supernatant was obtained; the supernatant was discarded, and the pellets were dissolved in NaOH (0.1 M, 1 mL). Finally, hemozoin content was determined by measuring the absorbance at 400 nm (Cecil spectrophotometer). The outcome was recorded as the percentage of inhibition (I%) of heme crystallization, employing DMSO as a negative control, using the following equation:
where An is the absorbance of the negative control, and As is the absorbance of test samples. Chloroquine diphosphate was used as the positive control in this assay (22).
3.5. TLC Analysis of Fractions
3.5.1. Preliminary Phytochemical Analysis of Fractions
The main chemical groups were identified using the TLC method on silica gel 60 F254 (Merck, Germany). Ethylacetate: Water: Formic acid: Acetic acid (100:26:11:11) and Chloroform: Methanol: Glacial acetic acid: Water (64:12:32:8) were used as the solvent system. Anisaldehyde sulfuric acid was used as the reagent, and visualization was performed under UV 366 nm after about 10 minutes at 100°C (23, 24). The Libermann- Buchard and Shinoda tests were performed to verify the results of TLC analysis.
3.6. Libermann-Buchard Test
The plant fractions were exposed to small amounts of acetic anhydride. The obtained mixture was boiled and cooled. Then sulfuric acid was added to the mixture from the sides of the test tube. The creation of a deep red color indicated the presence of triterpenoids (25).
3.7. Shinoda Test
Magnesium ribbon was added to the test sample, and then hydrochloric acid was added. The emergence of a crimson red, pink scarlet, or sometimes green to blue color after a few minutes showed the existence of flavonoids (25).
4. Results
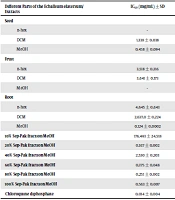
Table 1 summarizes the results of the cell-free beta-hematin formation test for three extracts (E. elaterium fruits, seeds, and roots) with different polarities and six fractions of the strongest extract (i.e., MeOH root extract).
| Different Parts of the Ecballium elaterium/ Extracts | IC50 (mg/mL) ± SD | IC90 (mg/mL) ± SD |
|---|---|---|
| Seed | ||
| n-hex | - | - |
| DCM | 1.338 ± 0.038 | 2.214 ± 0.069 |
| MeOH | 0.458 ± 0.094 | 2.703 ± 0.063 |
| Fruit | ||
| n-hex | 3.518 ± 0.316 | 4.332 ± 0.487 |
| DCM | 3.641 ± 0.173 | 20.414 ± 1.958 |
| MeOH | - | - |
| Root | ||
| n-hex | 4.645 ± 0.643 | 6.606 ± 1.191 |
| DCM | 2.637.0 ± 0.224 | 9.078 ± 1.286 |
| MeOH | 0.124 ± 0.0002 | 1.471 ± 0.009 |
| 10% Sep-Pak fraction MeOH | 176.493 ± 24.518 | 871.356 ± 130.730 |
| 20% Sep-Pak fraction MeOH | 0.167 ± 0.002 | 0.433 ± 0.005 |
| 40% Sep-Pak fraction MeOH | 2.530 ± 0.203 | 6.050 ± 1.109 |
| 60% Sep-Pak fraction MeOH | 0.375 ± 0.048 | 1.619 ± 0.018 |
| 80% Sep-Pak fraction MeOH | 0.251 ± 0.002 | 0.922 ± 0.004 |
| 100% Sep-Pak fraction MeOH | 0.563 ± 0.007 | 1.001 ± 0.019 |
| Chloroquine diphosphate | 0.014 ± 0.004 | 0.164 ± 0.006 |
The IC50% and IC90% Values of the Extracts and Sep-Pak Fractions of the MeOH Root Extract of Ecballium elaterium in the Cell-free Beta-hematin Formation Assay
Using the beta-hematin formation assay, the inhibition concentration (IC50%) and its standard deviation (SD) were determined for each sample (n = 3). The IC50 and IC90 values were calculated graphically by plotting the concentration against the inhibition percentage. Chloroquine diphosphate (i.e., with the most potent antimalarial effect) was used as the positive control and DMSO (i.e., without any antimalarial effect) as the negative control. In terms of seed extracts, although the DCM and MeOH extracts showed significant antimalarial activity compared to the control group (P < 0.001 and P < 0.0001, respectively), no antimalarial activity was observed for the n-hex extract (Table 1). Furthermore, the n-hex and DCM fruit extracts showed moderate antimalarial activity, whereas the MeOH extract could not inhibit hemozoin formation (i.e., no or negligible activity). Finally, different root extracts illustrated significant inhibitory activities compared to the control group (P < 0.001).
Among all the extracts, the IC50 of MeOH root extract was the lowest. Moreover, it considerably (P < 0.001) inhibited the transformation of heme to hemozoin compared to the control, as well as the DCM and n-hex root extracts and MeOH seed extract (with a minimum IC50). Hence, MeOH root extract was considered for fractionation. Among the obtained SPE fractions, although all of them revealed antimalarial activity compared to the control group (P < 0.001), the 10% SPE fraction could not notably inhibit hemozoin production.
The results of the TLC analysis of the most potent antimalarial fractions have been shown in Table 2.
| Fractions | Identified Chemical Groups |
|---|---|
| Fr.20% | Flavonoids (brown spots) |
| Fr.60% | Flavonoids (brown zones) |
| Fr.80% | Flavonoids (brown spots), Triterpenoids (violet black zones) |
| Fr.100% | Flavonoids (brown spots), Triterpenoids (violet spots) |
TLC Analysis of the Most Potent Antimalarial Fractions
5. Discussion
Malaria is an infectious life-threatening ailment caused by the bites of Anopheles mosquitoes. The mosquito (an intracellular parasite of the genus Plasmodium) (26) infects the host’s erythrocytes and utilizes hemoglobin as a nutrient source by degrading this protein to amino acids, which are used for energy production, growth, and maturation. Previous reports revealed that up to 80% of the host’s hemoglobin is degraded by these microorganisms (27). Hemoglobin degradation by protease enzymes releases the heme (ferrous) that is then oxidized to the ferric form. The latest form is toxic to the parasite and can damage biological membranes and suppress enzymes such as proteases. The parasite, which requires a mechanism to detoxify the released heme, has solved this problem by transforming the ferric heme to an insoluble, inactive, and crystalline form named hemozoin (i.e., the malaria-pigment) (28). Thus, inhibiting hemozoin formation can be a potential therapeutic method for this disease.
Scientific reports revealed the efficacy of several medicinal plants (e. g., different species of Artemisia, Cinchona, Cryptolepis, and Tabebuia genera) and their secondary metabolites in the treatment of malaria and pointed out their advantages over modern remedies (e.g., low cost, fewer side effects, etc.). For example, chloroquine is a principal natural anti-malaria medication derived from quinine (29).
In this study, nine different extracts of various parts and six fractions of the MeOH root extract of E. elaterium were assessed for their antimalarial activities in vitro. Among the different extracts assessed, the MeOH seed and root extracts (with the minimum IC50 of 0.458 ± 0.094 and 0.124 ± 0.0002, respectively) considerably inhibited heme to hemozoin transformation compared to the control (DMSO) (P < 0.001).
Although different studies have noted that the main ingredients of plants such as sesquiterpenoids and tri-terpenoids (Cucurbitacin E and α-Elaterin) (30), as potent antimalarial agents (31), are accumulated in fruits, we here observed that the antimalarial activity of the MeOH fruit extract was negligible (a high IC50) compared to the MeOH root extract.
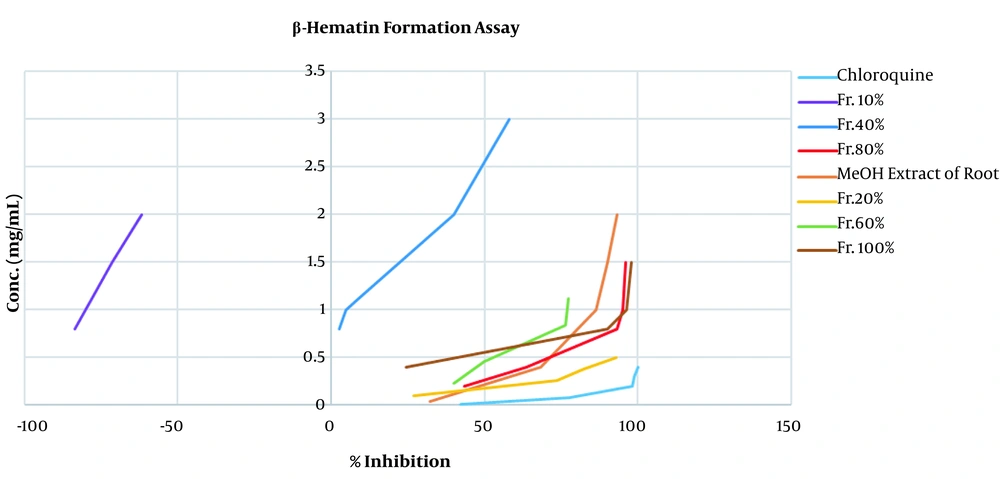
According to the obtained results, we performed the fractionation of the extracts that potentially had potent antimalarial components with high polarities using the SPE method on a CI Sep-Pak cartridge with solvent combinations with reducing polarities (21). All of the SPE fractions with different concentrations, except for the 10% fraction, inhibited the formation of hemozoin compared to the control group (P < 0.001). Furthermore, among the fractions, the 20% SPE fraction obtained the minimum IC50. Other fractions (80%, 60%, and 100%) also were distinguished with significant and potent antimalarial activity (P < 0.001) (Figure 1).
A preliminary phytochemical analysis of the most potent antimalarial fractions was performed using the TLC method. In addition, the Libermann-Buchard and Shinoda tests were applied to confirm TLC analysis results. The findings suggested the presence of flavonoids in the 20% and 60% fractions and flavonoids and triterpenoids in the 80% and 100% fractions.
Previous phytochemical investigations on E. elaterium demonstrated that the plant is a rich source of phenolic compounds such as flavonoids, flavanols, tannins, and carotenoids, as well as proteins triterpenoids, and lipids (32, 33). Cucurbitacin derivatives with triterpenic structures are among the main phytochemicals of E. elaterium and have been investigated for their anti-proliferative, anti-cancer, anti-inflammatory, and antimicrobial properties (34, 35).
According to previous studies, flavonoids and triterpenoids can be responsible for potent antimalarial effects (36, 37). For example, Cucurbitacin glycosides from Datisca glomerata (C. Presl) Baill showed antiplasmodial activities (38). Moreover, earlier studies showed that flavonoids had potential synergistic anti-malaria effects with artemisinin (the main antimalarial constituent of Artemisia annua L.) (39). Considering our findings and those of previous studies, it is necessary to focus on E. elaterium pure ingredients and investigate their biological (such as antimalarial) effects.
5.1. Conclusions
Out of the nine extracts with different polarities of E. elaterium different parts, the MeOH root extract was the most potent part in terms of the IC50 obtained in the cell-free beta-hematin formation assay. A primary phytochemical analysis on the SPE fractions of the most effective extracts suggested the need for purifying their active constituents and investigating their biological effects in animal models.