1. Background
Stabilization of blood sugar in mammalian cells is a crucial factor for life preservation. Physiologically, the β-cells of pancreas regulate blood glucose as a form of energy by insulin secretion. Thus, it is stated that these cells can control the state of primary metabolism in all parts of the body (1). In cancer therapy’s primary stages, high-energy radiation is applied to cancer cell death and inhibition of cancer cell proliferation (2).
It has been demonstrated that the healthy cells surrounding tumor mass can be affected by the fatal consequences of radiation. Besides radiotherapy, chemotherapy in chronic administration is able to increase the serum glucose level (causing diabetes status) (3). Also, according to some published papers, oxidative stress is considered a primary factor in pancreatitis etiology (4). In elevated levels of oxidative stress, the adverse biochemical process like lipid peroxidation occurs. In each stage of lipid peroxidation, a large amount of oxidative stress is formed, especially in the cell membrane, cell lipoproteins, and the membrane of organelles. This detrimental process leads to cell toxicity and deactivation of enzymatic antioxidant through two main intermediates; peroxidation and hydroxylation (5). Basically, there are two sources of oxidative stress production, namely endogenous and exogenous sources. Malformations of vital cellular proteins can easily lead to oxidative stress, a status that is known as endogenous sources. Other environmental stimulants, which alter the cellular genomic profiles or deactivate the intracellular enzymes, can lead to the accumulation of oxidative stress. These environmental stimulants (like radiation) are considered exogenous sources affecting pancreatic cells (6). The pancreas is a radiosensitive organ to chronic exposure of γ-ray (7). In this pathologic condition, the occurrence of mutagenesis, altered number of Langerhans islets, and eventually, apoptosis are possible (8). In response to γ-ray exposure, diabetes occurs through impairment of insulin secretion (9). Recently, the mice exposed to a low dose of radiation (0.1 Gy) can disrupt the pancreatic cells (10). It is essential to implement the application of natural radioprotectors in cancer treatments. These natural radioprotectors exert their protective roles by various mechanisms, including free radical scavenging, electron transferring, DNA repairing, and secretion of immune components such as inflammatory cytokines (11). Their therapeutic mechanisms are mainly based on antioxidant properties as a secondary protection system (12).
The Allium jesdianum (AJ) plant, with proved pharmacological properties, is a native herbal product of Middle East regions (13). The antioxidant features of the biochemical components of AJ (like flavonoids and phenolic) are applied by activation of white blood cells in response to superoxide produced via NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) (14). This plant is also used in traditional medicine to relieve acute gastrointestinal pains and rheumatoid arthritis (15). Hosseini et al. (16) indicated the supportive/regenerative influences of phytochemicals on pancreatic β-cells damages. In their extensive investigation, they found some critical therapeutic features of medicinal plants, including; Abroma augusta, Annona muricata, and Anastatica hierochuntica, which potentially elevate the number of beta cells, Alchornea cordifolia, Anacardium occidentale, Amaranthus spinosus, and Amaranthus caudatus that can potentially induce regeneration of β-cells, Allium sativum, which can increase the islets’ diameter and finally, the Azadirachta indica, which can increase the density of β-cells (16).
Considering the oxidation effects of γ-ray, and antioxidant properties of AJ, as a natural radioprotective agent, the probable therapeutic effects of hydroalcoholic extract of AJ against pancreatic γ-ray-related damages were assessed in the current study.
2. Objectives
In this study, the effects of Allium jesdianum on pancreatic toxicity induced by γ-radiation were investigated.
3. Methods
3.1. Experimental Animals
Sixty-four male mice (NMRI) with weight of 30 ± 5 g and the age of 12 - 14 weeks were acquired from the animal house (Kermanshah University of Medical Sciences,). The standard living conditions were prepared as; 12:12 hours light/dark cycle and 22 ± 2°C room temperature. Experimental investigations were applied based on the ethical and human principles of research. All administrations were approved by the Ethics Committee of Kermanshah University of Medical Sciences (code: IR.KUMS.REC.1398.298).
3.2. Plant Collection
The fresh stems and leaves of Allium jesdianum were collected (April 2019) from local areas of Ravansar (Kermanshah, Iran). They were authenticated by Dr. F. Firozian (Department of Botany, K. U. M Institute, Kermanshah, Iran). The plant specimen was put at K. U. M Institute with voucher number (no.: 2252) for further references.
3.3. Provision of AJ Extract
Waste components of the plant (else stems and leaves) were removed. The stems and leaves were dried in shadow for a week. The plant was ground, and 200 g of which was dissolved in 2,000 mL (1 - 10 wt/vol) of 70% ethanol. After two days of preservation in a hot water bath (35°C) and dark environment, the solution was poured on Buchner funnel filter paper vacuum pump device. The solution was transferred to a spinning system to increase the concentration of the external solvent. The extract was sterilized by the 0.2 µm filter (17).
3.4. Animal Groups and Administration Protocols
All animals were categorized into 8 groups (n = 8) randomly. The first group received normal saline as a control. Second, as γ-ray group received the dose of 2.5 Gy/min of gamma exposure. Third to fifth, as AJ groups, received various doses of AJ (500, 1,000, and 2,000 mg/kg, respectively). Sixth to eight, as AJ + γ-ray groups, received different doses of 500, 1,000, and 2,000 mg/kg of AJ + 2.5 Gy/min of gamma. All drug solutions with various concentrations were administrated orally (gavage). Also, extracts and γ-radiation were treated once a day for 70 consecutive days (12, 13).
3.5. Radiation Features
The Cobalt-60 machine (Theratron, 78°C) available in the cobalt therapy department was hired as a source of irradiation. Mice were irradiated with the following conditions; well-ventilated restrainer, whole-body exposure to γ-ray (2Gy), the dose rate of 1Gy/min, Calimator to isocenter distance (CID) of 30 cm, and a source-to-surface distance (SSD) of 80 cm (12).
3.6. Animal Tissue Sampling
For the tissue sampling procedure, the animals were anesthetized (by intraperitoneal injection of 40 mg ketamine). A 5-mL syringe was used for venous blood aspiration from the right ventricle of heart. The blood sample was incubated (15 min, 37°C), and the clot was centrifugated (3,000 rpm, 15 min) to separate the blood serum. For the future biochemical analysis, the fresh frozen (-70°C) serum sample was used. Finally, the cervical dislocation procedure was applied based on the standard research laboratory protocols. The pancreas tissue was dissected and stabilized in 10% formalin solution for microscopic investigations (5).
3.7. Serum Level of Nitrite Oxide
According to the colorimetric approaches, the Griess assay was applied for the measurement of nitrite oxide levels. The serum was deproteinized using 5 mg of zinc sulfate added to 500 µL of serum (1:100 zinc sulfate/serum) followed by centrifugation (12,000 rpm, 12 min). The supernatant was separated and mixed with the same volume of Griess reagent available in the wells of 96-well ELISA plates. The Griess reagent was made of sulfanilamide (1%) and naphtylethylenediamide (0.1%) dissolved in phosphoric acid (2.5%). This mixture was placed in an incubator (37°C) for 10 min. The absorbance was measured at 450 nm using a microplate reader (18).
3.8. Lipid Peroxidation Levels
The level of molecular reaction among thiobarbituric acid and malondialdehyde (MDA) following a colorimetric process was considered lipid peroxidation status. In this process, the frozen sample of pancreas tissue was used. First, the tissue was washed with phosphate-buffered saline at pH of 7. Then, an ultrasonic homogenizer in the cold phosphate buffer (containing ethylene diamine tetra-acetic acid) was hired to apply tissue homogenization. Twenty µL of supernatant was mixed within the test tubes (test tube had 4 µL of butylated hydroxytoluene, 20 µL of phosphoric acid (1 M), and 20 µL of TBA (thiobarbituric acid) solution). The test tube was incubated (60 min, 70°C) and centrifuged (10,000 rpm, 4 min). Then, 80 µL of the supernatant was poured into the spectrophotometer tubes. The produced dye in the commercial kit was read at 532 nm. Finally, the MDA level was presented as nmol/mg of protein (19).
3.9. Determination of FRAP
The FRAP is a method to determine the total antioxidant potential of the pancreas homogenate. This method is based on the modification of ferric reducing ability of plasma. The FRAP reagent is a mixture of 5 mL of 2,4,6-tripyridyl-s-triazine (10 mmol/L) in 50 mL of acetate buffer (pH 3.6, 0.1 M), 5 mL of ferric chloride (20 mmol/L), and hydrochloric acid (40 mmol/L) with the ratio of 25:2.5:2.5 (preheated to 40°C for 20 min). Subsequently, 100 µL of a pancreas homogenate was mixed with 50 µL of reagent and the reaction. Then the mixture was centrifuged (12,000 rpm, 20 min), and the supernatant absorbance was read at 593 nm (9).
3.10. Measurement of Serum Levels of Insulin and Blood Glucose
In three steps, the blood glucose was measured as follows: (1) The first day (baseline); (2) three days after the end of the treatment; (3) after the end of extract treatment. The animals were kept under malnutrition for 24 hours to assess fasting blood sugar (FBS). Just after blood aspiration, the sample was incubated for coagulation (20 min, 37°C). The blood coagulation was centrifuged (20 min, 4,000 rpm), and the serum isolated was held at -20°C. The glucose oxidase (GOD-PAP) technique was applied to assess serum glucose levels. Also, serum insulin was calculated by PLR ELISA (USA, Sigma) mouse kit (19).
3.11. Histological Observations
Fixed pancreatic tissue (in 10% formalin) was used for routine histological process (automatic tissue processors device). Finally, the paraffined tissue blocks were prepared, and 5-µm histological sections were cut by a microtome (Leica, Germany) and stained with hematoxylin and eosin. All histological assessments of stained slides were examined with an Olympus microscope. A director of photography 12 (DP12) Camera with a 3.34-million-pixel resolution connected to a microscope was applied to prepare the histological captures. Finally, the slides were analyzed morphometrically by Olysia Bio software (17).
3.12. Morphometric Measurements
Morphometrical assessments were applied to determine the number and diameter of pancreatic islands. For this purpose, 10 cuts from each tissue sample were studied morphologically by two separate laboratory experts (to reduce the probable measuring bias). Five fields in each cut were randomly selected and observed (100×). Thus, the mean value of each index was obtained. Also, the largest and smallest diameter of each islet was considered, and the mean diameter of islets was determined by the following formula; MD =
3.13. Real-time PCR
This method was used to evaluate the expression of the apoptotic genes, including p53 and Bax. The related sequences of primers used for gene detection are listed in Table 1. All pancreatic tissues were stored in a freezer (-80°C) to prevent tissue degeneration. Total RNA contents of pancreatic islands were extracted using the RNeasy mini kit (Qiagen Co.). Moreover, cDNA was produced from extracted RNA utilizing RevertAidTM First Strand cDNA Synthesis Kit (Fermentas). Also, DNA samples were treated by DNase set kit (Qiagen). The expression level of apoptotic genes was measured using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer as endogenous control by SYBR Green by the comparative method (20).
| Primer ID | Primer Sequences |
|---|---|
| GAPDH | F: 5’-AAGCTCATTTCCTGGTATG-3’ |
| R: 5’-CTGCCACAAGAACTAGAGA-3’ | |
| P53 | F:5’-AGAGACCGCCGTACAGAAGA-3’ |
| R:5’-GCATGGGCATCCTTTAACTC-3’ | |
| Caspase-3 | F: 5’-ATGGCGAAATGGAGATGAATA-3’ |
| R: 5’-ACTGCCCATGATGGTTCTGTG-3’ |
Primers Used in Real-time PCR
3.14. Evaluation of Inflammatory Cytokines
The ELISA method was used to assess the Toll-like receptor 4 (TLR4) (MyBioSource, California, USA), Interleukin-1 beta (IL-1β) (Abcam Cambridge, UK), and tumor necrosis factor-alpha (TNF-α) (Abcam, Cambridge, UK). Total proteins of pancreas were lysed by RIPA (Abcam, Cambridge, UK) and centrifuged at 15,000 g for 30 min. The ratio of 1:20 supernatants/dilutions were seeded into coated microplates with antibodies to induce enzyme-substrate reaction. Standard solutions were used for drawing standard curves. The amounts of proteins were examined in supernatant fractions by ELISA kits. The absorbance rate was measured in a triplicate manner at 450 nm (5).
3.15. Statistical Analysis
The Kruskal-Wallis test was used to examine data normality and the homogeneity of variance at a significance level of 0.05. For statistical analysis, one-way analysis of variance (one-way ANOVA) was used. Tukey post hoc test was applied to detect differences among various groups. All statistical analyses were performed using SPSS 16 software. The results were expressed as mean ± standard error, and P < 0.05 was considered statistically significant.
4. Results
4.1. Levels of FRAP, Lipid Peroxidation, and Nitrite Oxide
The FRAP levels of pancreas tissue in γ-ray group were significantly lower than the control group (P < 0.05). In both AJ and γ-ray + AJ groups, the FRAP level was increased considerably compared to the γ-ray group (P < 0.05). Moreover, γ-ray increased the levels of nitrite oxide and lipid peroxidation significantly in γ-ray group compared to the control group (P < 0.05). All doses of AJ with its therapeutic effects significantly decreased the mean levels of serum nitrite oxide and lipid peroxidation in AJ and γ-ray + AJ groups in comparison to the γ-ray group (P < 0.01) (Table 2).
| Groups | Nitrite oxide, mmol/mL | FRAP, mmol/mL | LP, nmol/mg |
|---|---|---|---|
| Control | 22.25 ± 1.5 | 51.65 ± 2.5 | 0.39 ± 0.01 |
| γ-ray | 110.36 ± 4.4b | 12.52 ± 1.2b | 0.8 ± 0.02b |
| AJ 500 mg/kg | 24.82 ± 3.6c | 58.36 ± 2.7c | 0.38 ± 0.02c |
| AJ 1,000 mg/kg | 22.11 ± 2.7c | 52.07 ± 3.8c | 0.35 ± 0.01c |
| AJ 2,000 mg/kg | 26.64 ± 3.8c | 51.65 ± 2.3c | 0.39 ± 0.03c |
| AJ + γ-ray 500 mg/kg | 62.73 ± 3.6c | 31.97 ± 3.1c | 0.51 ± 0.02c |
| AJ + γ-ray 1,000 mg/kg | 62.91 ± 2.4c | 34.11 ± 2.7c | 0.43 ± 0.03c |
| AJ + γ-ray 2,000 mg/kg | 160.05 ± 3.6c | 34.04 ± 1.9c | 0.42 ± 0.01c |
Antioxidant Parameters in the γ-Ray and AJ Groups (N = 8 for Each Group)a
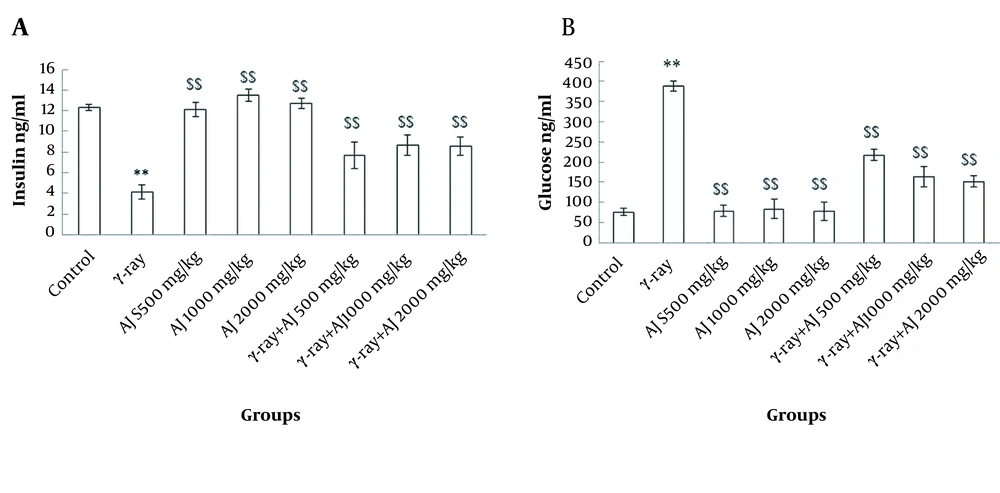
4.2. Serum Levels of Insulin and Blood Glucose
These values indicated a significant reduction in the level of insulin in γ-ray group and an incremental trend in glucose level compared to the control group (P < 0.05). Moreover, in AJ and AJ + γ-ray groups, significant growth of insulin level and a decline in glucose level were detected compared to the γ-ray group (P < 0.05) (Figure 1).
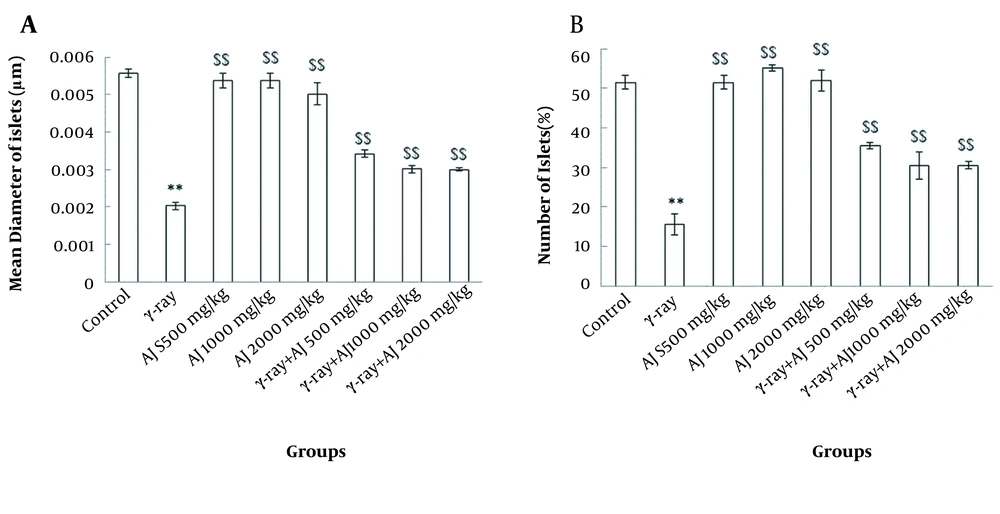
4.3. Morphometrical Variations
Two morphometrical values, including diameter and number of islets, revealed a significant decrease between the control and γ-ray groups (P < 0.05). Also, no significant differences were distinguished in morphometric variations in the AJ group compared to the control group (P > 0.05). Additionally, in AJ and γ-ray + AJ groups, a significant increase was seen in comparison to γ-ray group (P < 0.05) (Figure 2).
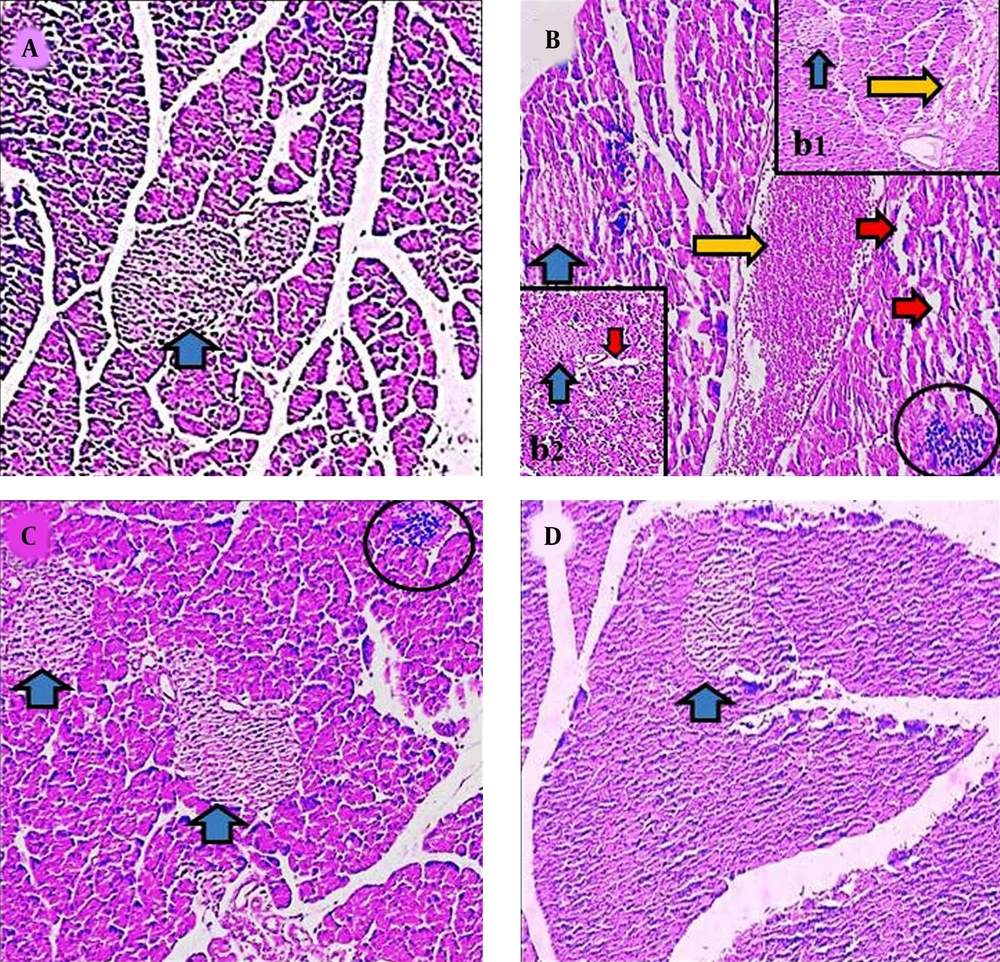
4.4. Histopathological Modifications
Histological investigation revealed that the structure of pancreas was altered in the γ-ray and AJ treatment groups. Following the exposure to the γ-ray (in the γ-ray group), the microscopical anomalies were seen that included vacuolization (indicating necrosis), reduction of islet, and blooding in pancreas tissue (indicating inflammation) compared to the control and γ-ray + AJ groups. Treatment with γ-ray + AJ (in all doses) concentrated the pancreas microscopically injuries caused by γ-ray (Figure 3).
Microscopic figures of pancreas tissue (4 µm, H & E, 100×). Micrograph of the pancreas section in the control groups (A), normal pancreas tissue. Micrograph of the pancreas section of γ-ray group (B, b1, b2), vacuolization in tissues (red arrow), reduction of islet size (blue arrow), and hyperemia (yellow arrow). Micrograph of the pancreas section in AJ group (2,000 mg/kg) (C), normal pancreas structure. Micrograph of pancreas section in AJ + γ-ray group (2,000 mg/kg) (D), normal pancreas tissues. AJ, Allium jesdianum.
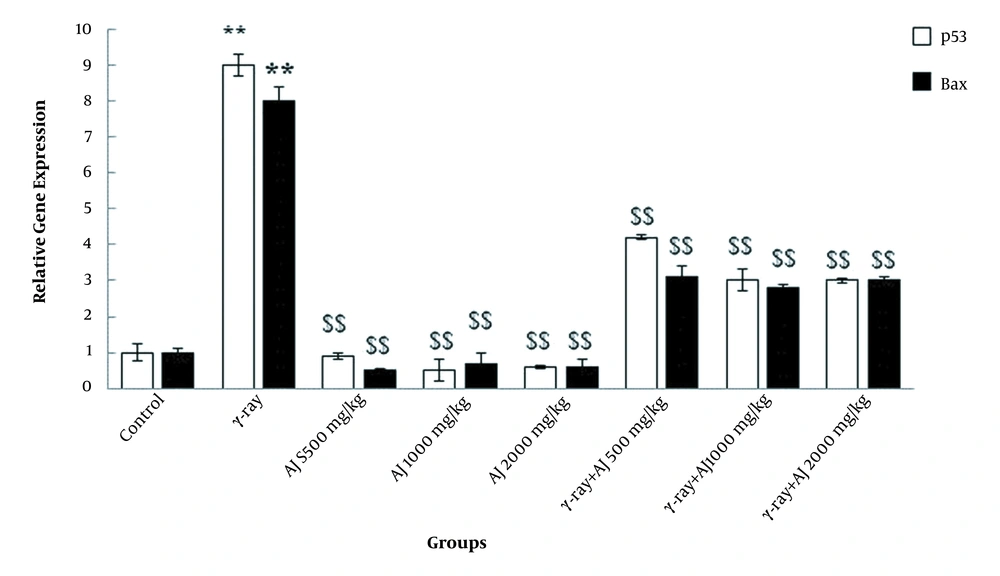
4.5. Gene Expression Levels
In this study, γ-ray, due to its detrimental impacts, significantly upregulated the apoptotic genes (p53 and Bax) in all members of the γ-ray group compared to the control group (P < 0.05). A significant downregulation of these genes was detected in all doses of extract in both AJ and γ-ray + AJ groups compared to the γ-ray group (Figure 4).
4.6. Levels of Inflammatory Cytokines
This value was significantly increased in the γ-ray group compared to the control group (P < 0.05). No significant differences were found in all AJ groups compared to the control group (P > 0.05). Additionally, whole several doses of AJ in AJ and AJ + γ-ray groups represented a significant decline in the inflammatory cytokines compared to the γ-ray group (P < 0.05) (Table 3).
| Groups | TNF-α, pg/mL | IL-1β, pg/mL | TLR4, pg/mL |
|---|---|---|---|
| Control | 63.52 ± 3.6 | 99.87 ± 8.3 | 72.12 ± 4.3 |
| γ-ray | 154.63 ± 7.1b | 251.41 ± 9.2b | 163.31 ± 7.1b |
| AJ 500 mg/kg | 67.28 ± 5.8c | 101.31 ± 6.7c | 75.82 ± 5.7c |
| AJ 1,000 mg/kg | 61.85 ± 6.3c | 98.73 ± 5.1c | 71.04 ± 5.1c |
| AJ 2,000 mg/kg | 63.10 ± 5.1c | 100.31 ± 7.3c | 73.64 ± 4.3c |
| AJ + γ-ray 500 mg/kg | 93.26 ± 3.6c | 131.01 ± 8.8c | 92.72 ± 6.2c |
| AJ + γ-ray 1,000 mg/kg | 84.63 ± 8.4c | 132.91 ± 3.4c | 93.54 ± 4.7c |
| AJ + γ-ray 2,000 mg/kg | 84.21 ± 4.2c | 129.83 ± 8.3c | 90.12 ± 3.3c |
Effects of AJ and γ-Ray on Pancreas Levels of TNF-α, IL-1β, and TLR-4
5. Discussion
Since the pancreas is involved in the regulation of blood glucose, it is considered a vital organ (19). Nowadays, the rate of cancer epidemy is higher than ever. In addition, radiotherapy is an essential part of cancer treatment. However, due to the side effects of radiation, there are no appropriate preventive approaches to deal with these complications. The herbal medicines have lesser side effects and high efficacy than other chemical agents. In order to overcome the side effects of radiotherapy, the plants are acceptable therapeutic alternatives (12). Thus, the possible radioprotective effects of AJ, as an antioxidant agent, were analyzed following exposure to γ-rays. In this research, the histological changes in the γ-ray group showed a substantial decrease in diameter and the number of Langerhan’s islets. In addition, the above-mentioned values were increased in all animals in γ-ray + AJ group in comparison to the γ-ray group.
Even in the acute phase, the γ-ray induces apoptosis via two main pathways; reactive oxygen species (ROS) production and nitrogen species (NOS) generation. With poor patients’ antioxidant protection system, these reactive species can lead to cell death (apoptosis) (21). The pancreatic beta cells, which influence blood sugar have impressive effects on this phenomenon. The attack of free radicals to pancreas can potentially induce inflammation in which the macrophages migrate to the islands of Langerhans. The resident macrophages initiate phagocytosis in pancreas, causing the lower islets size and insulin release (22). Based on the findings of Son and coworkers, this fatal process is aggravated by the presence of peroxide nitrite in islets of Langerhans (23). Following oxidative stress, AJ initiates an anti-inflammatory process through nuclear factor erythroid 2-related factor 2 and heme-oxygenase1expression (24). In addition, the findings of these studies indicated that AJ extract could potentially inhibit cyclooxygenase-2 gene expression in streptozotocin (STZ)-induced pancreatic tissue of diabetic rats (25). Allium jesdianum stimulates the inflammatory gene expression to prevent pancreatic injuries following morphine usage (14). Furthermore, as a consequence of γ-ray exposure, it seems that lipid peroxidation contributes to ROS development and free radicals generation. In fact, ROS targets saturated fatty acids and induces protein alkylation (13). These adverse events in the pancreatic cells contribute to pancreatic inflammation and mononuclear inflammatory cell infiltration (26).
In this analysis, the AJ was found to be able to decrease lipid peroxidation and increase the ability of antioxidant activity. Inconsistent with these results, many studies have demonstrated the antioxidant characteristics of AJ (13, 14). As a result, AJ inhibits lipid peroxidation and increases FRAPS levels (27). It also controls the inflammatory pathway that completely inhibits the mechanism of apoptosis (15). The findings of this study have been confirmed by Salahshoor et al. (19), which established the property of genistein to avoid cytotoxicity caused by oxidative stress. They found that this phenomenon could lead to substantial reduction in diameters of the central vein of liver and the normal size of hepatocytes. Also, bromobenzene was contributed to cell toxicity. Kalantari (13) concluded that the administration of AJ could lead to reduced levels of MDA, confirming the findings of this report. In the γ-ray group, the serum levels of insulin and glucose were decreased and increased, respectively. Increased levels of insulin and decreased levels of glucose in all research groups were seen using AJ and γ-ray (in the AJ + γ-ray groups). Rafiey et al. (17), in confirmation of the findings of this report, stated that the hydroalcoholic extract of Falcaria vulgaris, by its therapeutic characteristics, could decrease the serum glucose levels and elevate insulin in diabetic rats induced by STZ. In this research, it was found that AJ has preventive properties on β-cells in which the insulin levels were reduced after γ-ray exposure in non-diabetic mice. Reduced secretion of insulin is probably related to the destruction of β-cells following radiation (9). By the antioxidant feature, AJ appears to decrease blood glucose levels, thus affecting pancreatic beta cells. This antioxidant can inhibit the death of beta cells, increased the level of insulin, while reduced the level of glucose (28).
Microscopic pathological changes of pancreatic tissue have been detected in the γ-ray group in this study. These pathological lesions included hemorrhage in pancreas, deformation of Langerhans islets, dilatation, and disruption of pancreatic acinar cells. In the case of histological disorders during γ-ray, the macrophages could influence cells principally due to the influx of intracellular enzymes of organelle into the intracytoplasmic matrix caused by loss of organelle membrane integrity and stability. In cell membrane damage, in which the release of intracellular enzymes happens, this negative phenomenon can be exaggerated (19). Upon medication by AJ, all abnormal alterations would be minimized because this extract showed antioxidant activities.
The elevated levels of nitrite oxide in blood serum were demonstrated in the γ-ray group. Nitrite oxide levels in animals of AJ + γ-ray group represented a significant reduction due to the antioxidant and anti-inflammatory nature of AJ. Nitrite oxide, as a common free radical in mammalian cells, interferes with physiological processes. Hydroxyl radicals are formed by nitrite oxide and superoxide anions in the pathogenesis procedure (5). The decline in nitrite oxide levels is attributed to the lower expression of iNOS. Antioxidant agents minimize the generation of nitrite oxides (18). According to the obtained data of Sohrabinezhad et al. (15), AJ is pharmacologically able to inhibit nitrite oxide-inducing lipopolysaccharide. They assumed that this molecular event is basically related to the induction of calmodulin-calcium-dependent kinase-4 protein expression (15). In addition, this research showed that AJ could reduce the expression of apoptotic genes (p53 and Bax). P53 changes the stability of the mitochondrial membrane, which induces the intracellular matrix inflow of cytochrome c. Thus, these apoptotic factors can control Bax and caspases activities (29). In addition, γ-ray stimulates apoptotic variables in pancreatic cells (30). AJ is moved into the intranuclear matrix to modulate the apoptotic factors (31). These genes were upregulated following β-ray exposure in experimental research on human lymphoblastoid cells performed by Fu et al. (32). In this analysis, it has been suggested that AJ has medicinal properties to reduce the expression of apoptotic genes caused by ROS. Thus, AJ is introduced as an antioxidant with a plant basis, which potentially eliminates pancreas-damaged through γ-radiation, including reduction of glucose level, enhancement of antioxidant capacity, and reduction of serum nitrite oxide.
In addition, our findings showed a substantial increase in the rate of inflammatory indicators in the γ-ray group. The interaction between nitrite oxide and inflammatory factors has been found in an investigation in the field of lung damage caused by lipopolysaccharide (33). AJ administration declined pro-inflammatory cytokines in pancreas; however, TNF-α had minor, while IL-6 showed higher activity rates (16). Consequently, a positive relationship was suggested among nitrite oxide levels, TLRs, and inflammatory cytokines as a result of the current study. However, in the inflammation phase, the prominent role of cell types in intercellular interchange between TLR and pro-inflammatory cytokines should not be abandoned. Also, we showed a high positive association between nitrite oxide and TNF-α, IL-1β, and TLR-4 expression in pancreatitis, which was reduced by AJ administration. This is considered a complicated event when Jalili and colleagues analyzed the collaboration between TNF-α, TLR-4, and IL-6 in the Acacetine-induced safety cascade in hepatitis subsequent ischemia-reperfusion (5). This study exclusively examined the probable relationship between IL-1β and TLR-4 or its dependence in the case of γ-radiation and AJ administarion. According to the results of this study, it is worth-noting that the doses of AJ are in high grade, which is not recommended for human exposure. Consequently, the dose and potential harmful effects on human cells should be taken into consideration according to the experiments of this study to protect β-cells in patients exposed to γ-radiation through this plant.
5.1. Conclusions
The outcomes of the current study displayed the potential effects of AJ (mainly antioxidant properties) against toxic effects of γ-ray. Due to the inherent toxicity of synthetic medicines in the presence of destructive ionizing radiation, this study focused on natural medications’ findings based on medicinal plants. AJ administration modulates the expression of apoptotic genes, inflammatory markers, serum glucose, and growth antioxidant agents. These properties can mediate antioxidant properties of AJ extract. The present study revealed that AJ is a potent radioprotector, which can be used as potential modern therapies. Though, more research in animal models is necessary to obtain more conclusive evidence for the molecular interaction between AJ and γ-ray, leading to recover pancreas destruction.