1. Background
As a chronic, progressive, and age-associated disease affecting the lung, idiopathic pulmonary fibrosis (IPF) is usually considered as the most common form of idiopathic interstitial pneumonia (IIP) (1). There are several risk factors for IPF, including cigarette-smoking, air pollution, aging, genetic factors, viral infections, etc. (2). In this disease, inflammation and fibrosis may occur in pulmonary interstitium, which is accompanied by the injury of lung epithelial cells, accumulation of fibroblasts and myofibroblasts, and deposition of extracellular matrix (ECM) components, leading to lung structural changes and pulmonary hypertension. Following that, respiration and gas exchange are impaired, culminating in a surge in morbidity and mortality rates (3). Because of unknown pathogenic mechanisms, the effective treatment of IPF is still among the greatest clinical challenges of the field.
Until now, there is no approved pharmacological therapy for relieving the disease’s symptoms and enhancing the survival of patients. In 2014, Pirfenidone and Nintedanib were approved as therapeutic agents for the disease, which reduce the forced vital capacity (FVC) and prevent disease progression (2).
Bleomycin (BLM), as a chemotherapeutic antibiotic, is produced by the bacterium streptomyces verticillus (4) and is used in studies on animal models of PF, which is among the major adverse effects of BLM during cancer therapy. The drug induces inflammatory reactions (3) and fibrotic changes within a short period of time after instillation (5).
As a yellow pigment present in an Indian spice, turmeric (Curcuma longa) or curcumin (diferuloylmethane) has shown potent anti-inflammatory, anti-cancer, and antioxidant effects, yet many preclinical trials are in progress to investigate its ability in preventing cancer and inhibiting inflammation (6).
Drug delivery through the pulmonary pathway is an interesting administration route that is performed via inhalation through the throat and trachea. As a straightforward pathway for delivering drugs to the lung, inhalation therapy is regarded as the most effective treatment for lung diseases such as PF, asthma, etc. (7).
2. Objectives
C. longa (turmeric)-derived curcumin has been extensively studied in the last decades for its numerous pharmacological activities including antioxidant (8), anti-inflammatory (9), anti-cancer (10), and antimicrobial (11) activities, as well as immunoregulatory (12) and antifibrotic (13) capacities. Nevertheless, curcumin has poor aqueous solubility and stability and unfavorable bioavailability because of its fast in vivo metabolism (13), making it less applicable in clinical applications. In this context, various nanotechnology approaches have been used to foster curcumin stability and absorption (14). Thus, in this study, we present a novel route for the administration of the nano-curcumin formulated by β-cyclodextrin in rats with BLM-induced PF.
3. Methods
3.1. Chemical Agents
Curcumin was prepared from Merck (Concord Rd, Billerica MA), and β-cyclodextrin was purchased from SAMCHUN (Sandan-ro, Pyeongtaek, South Korea). Prednisolone was obtained from Aburaihan Pharmaceutical Company (Tehran, Iran). Bleomycin (injectable, CELON laboratories, Telangana, India) was purchased from Imam Sajad Pharmacy (Ahvaz, Khuzestan Province, Iran).
3.2. Animals
Male Sprague-Dawley (SD) rats (weight of 170 - 220 g) were purchased from the Animal House of Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Khuzestan Province, Iran). They were maintained in the standard condition with free access to ad libitum water and standard rodent laboratory chow. The study’s experimental design was approved by the Institutional Animal Ethics Committee (IAEC) of Ahvaz Jundishapur University of Medical Sciences (N-9707). The experiment was done in compliance with the guide for the care and use of laboratory animals of the National Institute of Health (NIH).
3.3. Pulmonary Fibrosis Induction
Rat models of PF were created according to previous studies (15). Bleomycin (5 IU/kg of body weight) was instilled intrathecally after the anesthetization of rats by the intraperitoneal (IP) injection of ketamine (75 mg/kg) and xylazine (5 mg/kg).
3.4. Experimental Design
The optimum dose of BLM (5 IU/kg) was chosen based on a preliminary study in which the drug appropriately induced PF without influencing the survival of animals. Eighty rats were randomly divided into eight groups: (1) saline + vehicle inhalation, (2) BLM + vehicle inhalation, (3) BLM + oral prednisolone, (4) BLM + oral curcumin, (5) BLM + curcumin inhalation, (6) BLM + nano-curcumin inhalation (50 µg/kg), (7) BLM + nano-curcumin inhalation (100 µg/kg), and (8) BLM + nano-curcumin inhalation (200 µg/kg). In the groups receiving curcumin, the agent was dissolved in distilled water and applied through nebulization for 21 consecutive days after BLM intratracheal administration. The route of administration, doses of curcumin, and the sample size were determined according to a previous study (16).
The rats were euthanized at the end of the experiment, and the entire lung was washed out with saline and then weighed. The lung index was calculated as the ratio of the lung wet weight (mg) to the body weight (g).
3.5. Preparation of the Nano-Formulation
Forty mg of β-cyclodextrin was dissolved in 8 mL of deionized water in a vial with a magnetic bar. Twelve mg of curcumin dissolved in 500 µL of acetone was added dropwise to the cyclodextrin solution while stirring at 400 rpm. The solution was stirred overnight, then passed through a 0.45 µm filter, and finally freeze-dried (17).
3.6. Characterization of the Formulation
3.6.1. Particle Size
For estimating particle size, 0.025 g of the formula was dispersed in 2.5 mL of distilled water, and then particle size was calculated after diluting the solution with water using the "Qudix" machine (Korea). Finally, the diameter of 90% of the particles was determined as 275 nm.
3.6.2. Determination of Curcumin Loading
First, 2.6 mg of the formulation was dissolved in 3 mL of acetone to extract curcumin using the solvent. It was stirred for 3 hours and then filtered to obtain the solution containing curcumin. Curcumin concentration was estimated by a standard UV-vis spectrophotometer at 423 nm (17). Following the same procedure, a standard plot for the curcumin acetone solution (0 - 12.5 µg/mL) was prepared.
3.6.3. Determination of Curcumin In Vitro Release
Curcumin powder was dissolved in the simulated lung fluid (SLF) containing tween 80, H2O, and polyethylene glycol (PEG) 4000 while stirring at 100 rpm and 37°C for 96 h. Then the suspension aliquot was piped at predetermined times before adding an equal volume of fresh SLF.
3.7. Cytokine Measurement
Cytokines’ concentrations in the lung tissue were measured by the enzyme-linked immunosorbent assay (ELISA) kits (Bioassay Technology Company, China) using the direct sandwich method.
3.8. Histopathological Examination
Lung tissues were fixed in a 10% buffered formaldehyde solution, and then tissue slices were embedded in paraffin and stained with hematoxylin and eosin (H & E) and Masson’s trichrome to prepare the tissues for histological evaluation.
3.9. Statistical Analysis
Statistical analysis was performed using PRISM version 8.0.2 software. Results were presented as means and ± standard error of the mean (SEM). Data were analyzed by one -way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. A P-value of < 0.05 was considered statistically significant.
4. Results
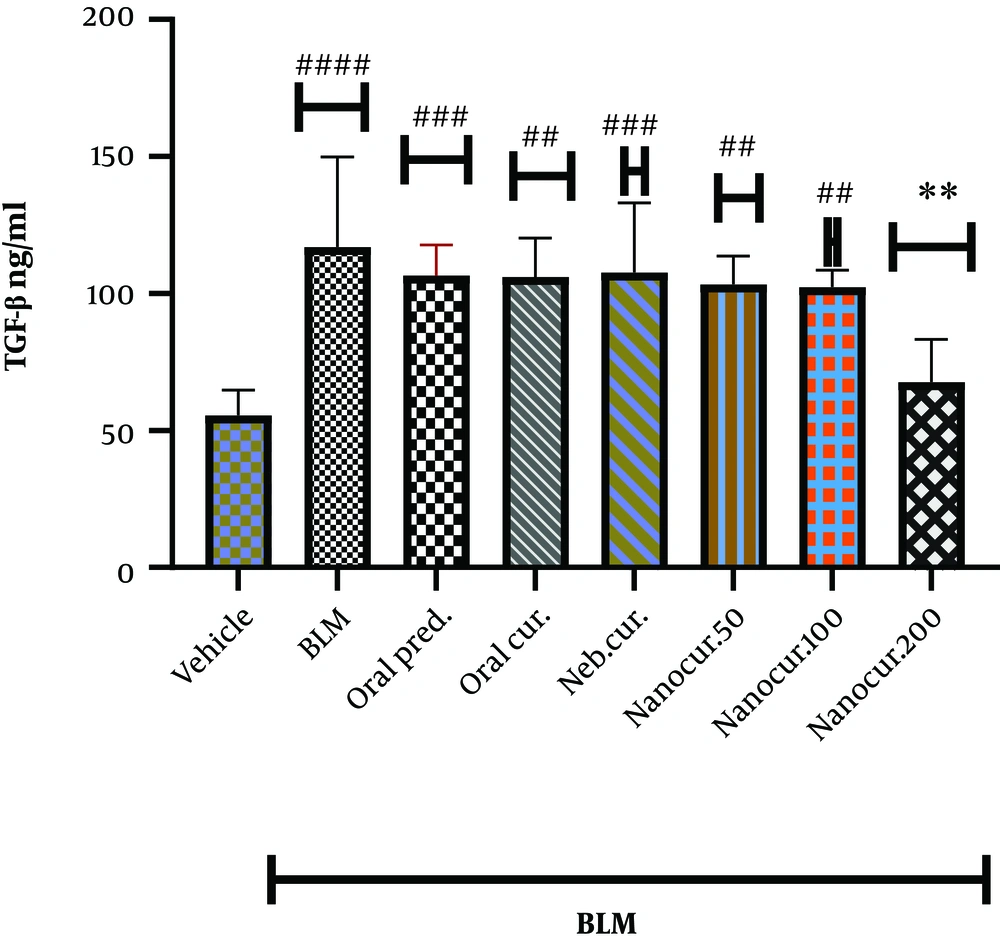
4.1. The Effects of Nano-Curcumin on Lung TGF-β Level
As shown in Figure 1, the intrathecal injection of BLM significantly increased TGF-β level in the lung (P < 0.0001). The inhalation of the nano-curcumin at the dose of 200 µg/kg, unlike the doses of 50 and 100 µg/kg, significantly reduced lung TGF-β level (P < 0.001). There was no significant difference comparing lung TGF-β concentrations between 50 and 100 µg/kg doses of the nano-curcumin.
The effects of the synthesized nano-curcumin at the doses of 50, 100, and 200 µg/kg on TGF-β concentration (ng/mL) in the lung tissues of the rat models of BLM-induced IPF. Values were expressed as means ± S.E.M. (n = 10) (* P < 0.05 compared to the BLM group; # P < 0.05 compared to the vehicle group).
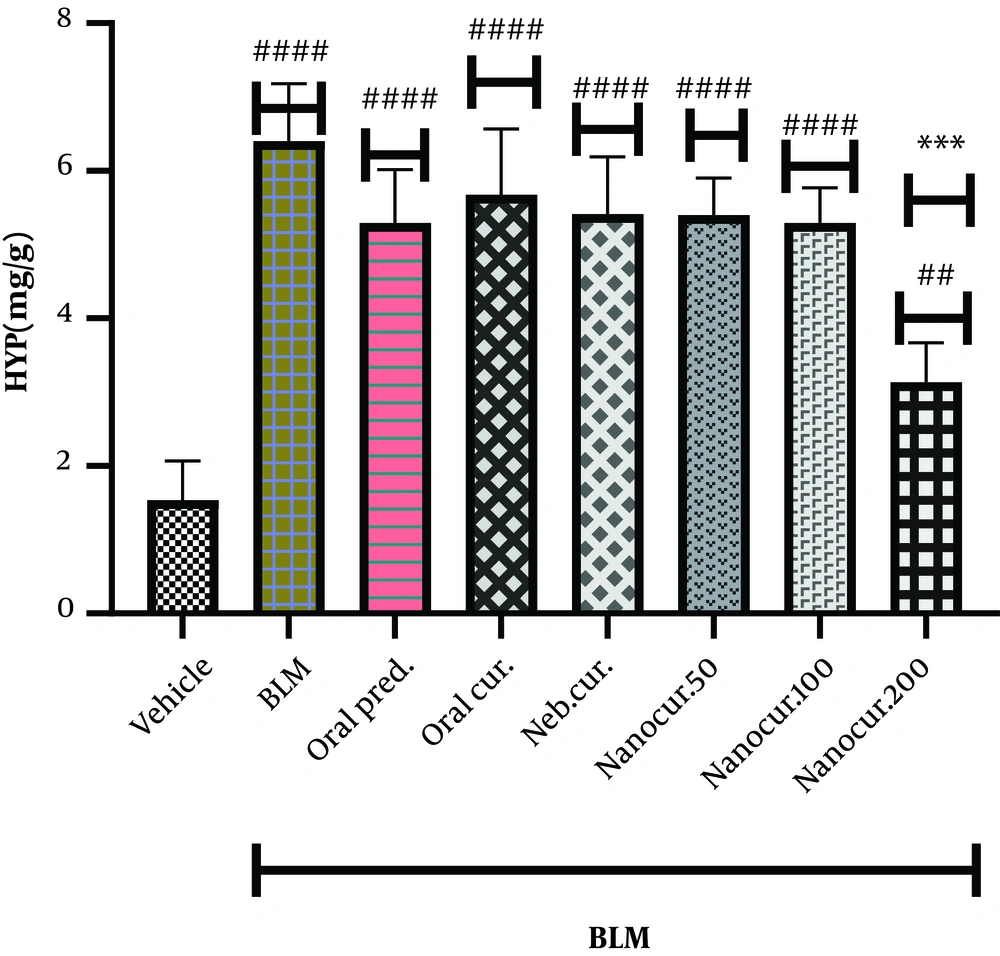
4.2. Effects of the Nano-Curcumin on Lung Hydroxyproline Level
As shown in Figure 2, the intrathecal injection of BLM significantly increased the level of hydroxyproline (HYP) in the lung tissue (P < 0.0001). Twenty-one days after nano-curcumin inhalation (the dose of 200 µg/kg) and in contrast to 50 and 100 µg/kg concentrations, there was a significant reduction in lung HYP level (P < 0.002). There was no significant difference comparing lung HYP concentration between 50 and 100 µg/kg concentrations. On the other hand, there was a significant difference between the treatment groups and the vehicle group comparing HYP levels.
The effects of the synthesized nano-curcumin at the doses of 50, 100, and 200 µg/kg on HYP concentration (mg/g) in the lung tissues of the rat models of BLM-induced IPF. Values were expressed as means ± S.E.M. (n = 10) (* P < 0.05 compared to the BLM group; and # P < 0.05 compared to the vehicle group).
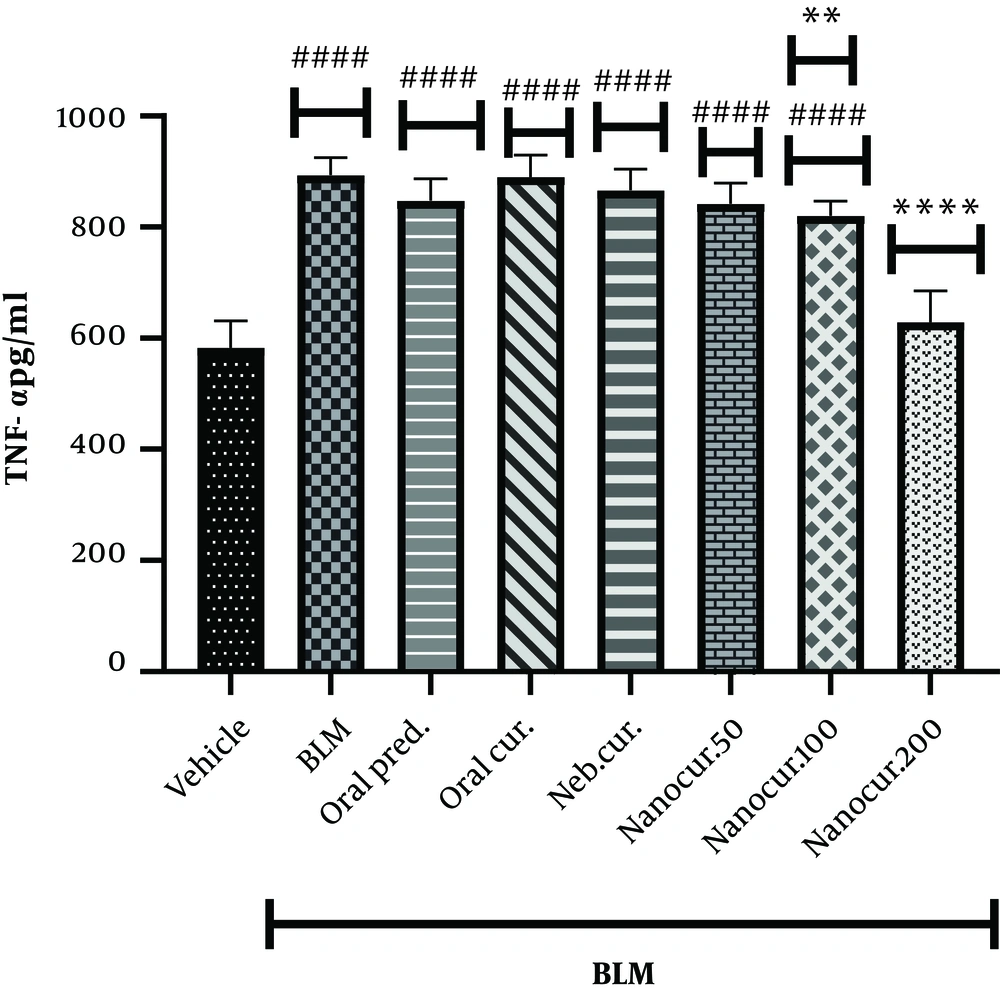
4.3. Effects of the Nano-Curcumin on Lung TNF-α Level
After 21 days of the nano-curcumin treatment, the lung concentration of TNF-α significantly reduced in the groups treated with 100 (P = 0.0016) and 200 µg/kg (P < 0.0001) of the nano-curcumin (Figure 3). On the other hand, BLM instillation significantly increased lung TNF-α level (P < 0.0001). Compared to the control group, lung TNF-α concentration significantly reduced in the groups treated with 100 and 50 µg/kg of the nano-curcumin while there was no significant difference between these two groups (P = 0.9742).
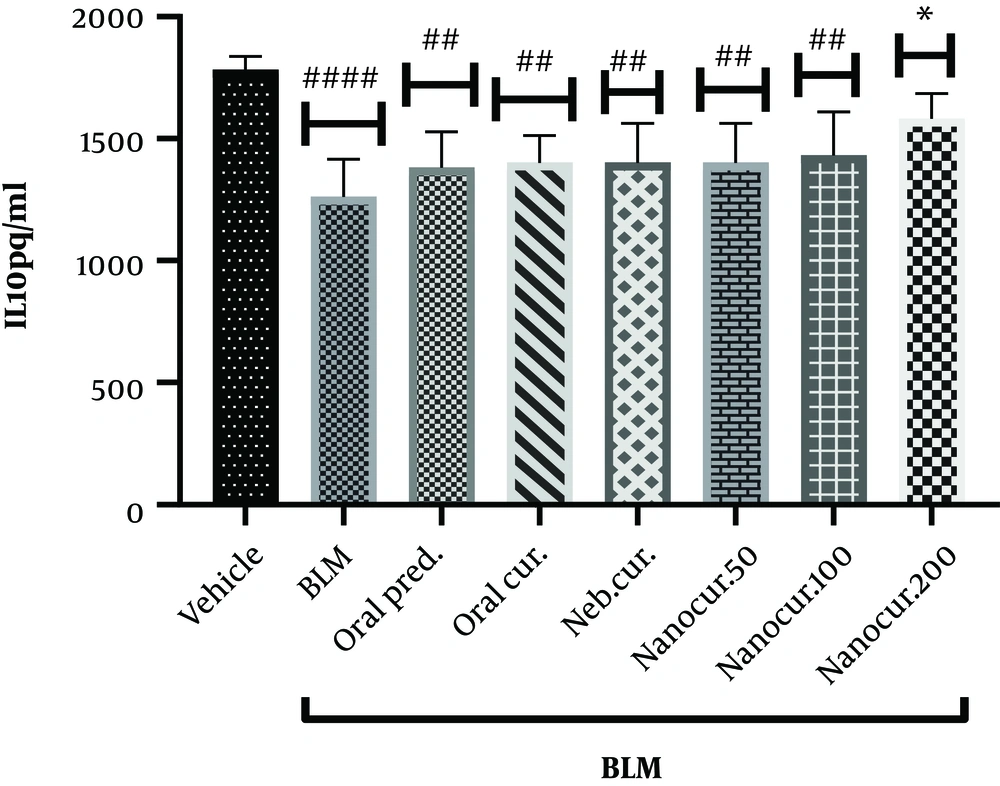
4.4. Effects of the Nano-Curcumin on Lung IL-10 Level
As shown in Figure 4, IL-10 level significantly decreased in the lung tissue following BLM injection (P < 0.0001) but significantly elevated after the inhalation of the nano-curcumin at the dose of 200 µg/kg for 21 days (P = 0.0144). There was no significant difference comparing lung IL-10 level between the groups receiving either oral prednisolone, oral curcumin, nebulized curcumin, or inhaled curcumin at the doses of 50 and 100 µg/kg.
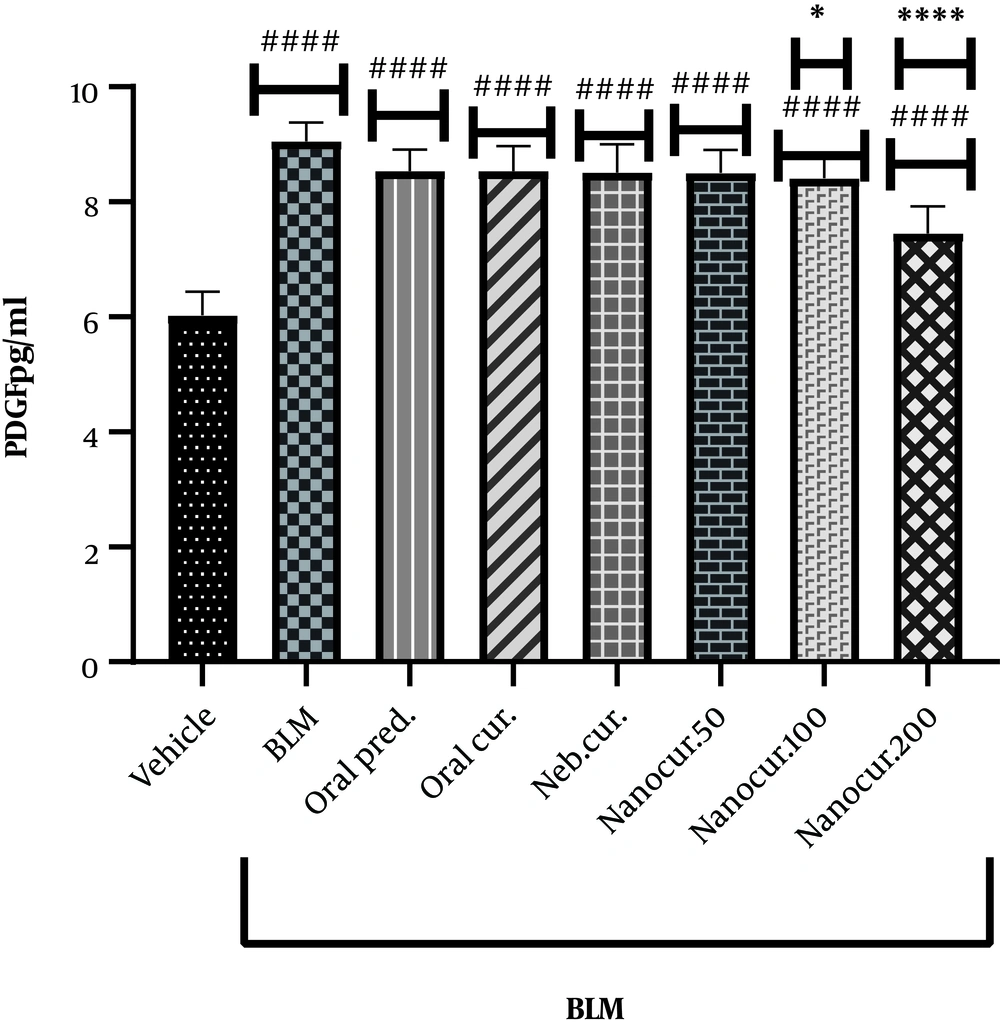
4.5. Effects of the Nano-Curcumin on Lung PDGF Level
The inhalation of the nano-curcumin for 21 days in the rat models of IPF (induced via the intratracheal injection of a single dose of BLM) significantly reduced lung PDGF concentration from 9.05 pg/mL (the BLM-treated group) to 7.45 pg/mL (the nano-curcumin inhalation group, 200 µg/kg) (P < 0.0001, Figure 5).
The effects of the synthesized nano-curcumin at the doses of 50, 100, and 200 µg/kg on lung PDGF concentration (pg/mL) in the rat models of BLM-induced IPF. Values were expressed as means ± S.E.M. (n = 10) (* P < 0.05 compared to the BLM group; and # P < 0.05 compared to the vehicle group).
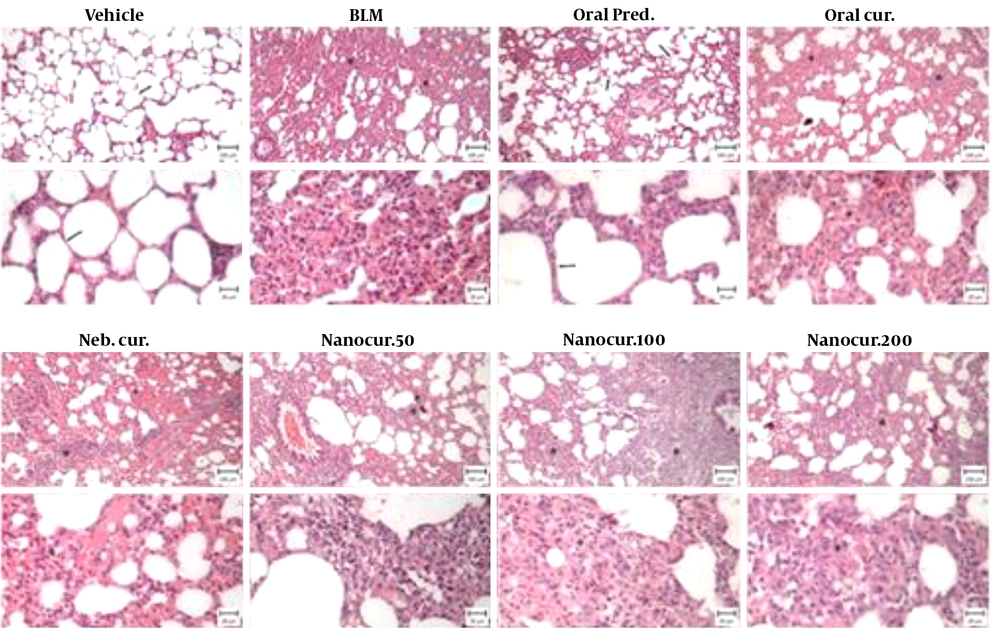
4.6. Histological Examination
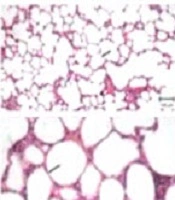
A normal respiratory tissue was observed in the vehicle group, characterized by an intact alveolar wall and no pathologic changes. In the BLM-treated group, severe pulmonary lesions including destroyed alveoli, thickened and edematous alveolar walls, and infiltrations by inflammatory cells, as well as the proliferation of fibroblastic cells associated with fibrosis were observed. These histopathological changes were also observed in the nano-curcumin treated groups. In the oral prednisolone-treated group, milder histological changes were observed, but still, there was inflammation (Figure 6).
Photomicrographs from the lungs of rats in eight different experimental groups (bar: 100 and 20 µm). In the vehicle (healthy control) group, intact alveolar spaces were seen within the context of a thin connective tissue (arrows). In the BLM-treated group, there were thickened alveolar spaces along with infiltrations by inflammatory cells. The mentioned histopathological changes were also observed in nano-curcumin treated groups. In the oral prednisolone-treated group, fewer histological changes were observed, but still, there was inflammation.
5. Discussion
Pulmonary fibrosis is a complicated and deadly disease, and inflammation has a dominant role in its pathogenesis. Despite numerous studies on the disease, a few drugs have been produced to effectively treat the condition. Animals with BLM-induced IPF, as perfect models, have been extensively studied to divulge the disease’s pathogenic mechanisms. Besides, curcumin, as a yellow chemical derived from Turmeric, has been shown to be a potent anti-inflammatory, anti-cancer, antifibrotic, and antioxidant substance (5). Curcumin can prevent inflammatory responses and also inhibit BLM toxicity against the alveolar epithelium (16). Curcumin has been effective in controlling the symptoms of various IPF animal models (rats and mice), including BLM–induced IPF models. In line with previous studies, the results of the present study showed that curcumin could block the release of TNF-α, HYP, TGF-β, and PDGF, as potent mediators of fibroblast proliferation and fibrotic changes, in the lungs of the rat models of BLM-induced IPF. Additionally, our results indicated that the inhalation of drugs (curcumin in this study), as an effective administration route, could probably enhance their therapeutic potential for IPF treatment. In this study, curcumin was directly delivered (i.e., inhalation) to the target tissue (the lung). Clearly, drug inhalation, as a targeted delivery approach, is more effective and has less side effects when used for treating pulmonary diseases. Accordingly, our findings revealed that the inhalation of the nano-curcumin formulated by cyclodextrin at the dose of 200 µg/kg could effectively treat IPF while such effects were not seen upon the oral administration of 1 mg/kg prednisolone.
Investigating the anti-inflammatory and antifibrotic mechanisms of curcumin, previous studies have shown that this agent can suppress the development and progression of BLM-induced inflammation through inhibiting TNF-α release from macrophages (18). TNF-α, as one of the most important biomarkers in inflammatory lung diseases, is significantly elevated during the progression of pulmonary fibrosis (19). In this study, similar to the recent research, TNF-α was significantly increased in the rat models of BLM-induced fibrosis while the animals that inhaled the nano-curcumin, especially at the dose of 200 µg/kg, showed a pronounced reduction in the level of this inflammatory marker. Bleomycin treatment significantly increased the HYP content of the lung by generating free radicals (20) while curcumin blocked the formation of toxic free radicals, which in turn could significantly decrease HYP production. Also, curcumin can modulate collagen metabolism (21). Unlike oral curcumin administration and curcumin inhalation, our data showed that the inhalation of the synthesized nano-curcumin could restore lung HYP content. On the other hand, curcumin could block TGF-β-induced differentiation of fibroblasts and collagen deposition by these cells (22) via downregulating the expression of this factor. This growth factor modulates the activation of inflammatory cells and fibroblasts and induces the production of ECM components and the differentiation of fibroblasts to myofibroblasts (13). Platelet-derived growth factor (PDGF) is an effective mitogenic and fibrogenic growth factor that stimulates collagen synthesis by fibroblasts (23). The signalling pathway triggered by PDGF in the fibrotic system is mediated through a number of fibrogenic factors such as TGF-β, TNF-α, etc. that show PDGF-dependent fibrotic activities (24). The role of IL-10 in PF pathogenesis is still unclear. In addition, IL-10 gene delivery has been shown to attenuate PF signs and symptoms, and some studies have also noted that the inhibitory effect of this cytokine is executed on the inflammatory, but not fibrotic, stage of the disease (25, 26). This study demonstrated that the inhalation of the nano-curcumin significantly decreased lung TGF-β and PDGF compared with the control group.
In conclusion, in agreement with the data of several previous studies, our results indicated that curcumin exerted anti-inflammatory and protective effects against BLM-induced PF in experimental rat models. Nevertheless, curcumin is insoluble in water, and delivering adequate quantities of curcumin to target cells and molecules is necessary for its potent protective or therapeutic effects (27). Smith et al. showed that the oral administration of curcumin had no effects on the treatment of IPF; however, the disease significantly improved following the intraperitoneal administration of this agent (28). This may be explained by the poor absorption of curcumin in the enteral route. The poor bioavailability of curcumin is the most important issue regarding the clinical use of this drug, necessitating the use of its soluble formulations, such as the forms incorporated with nanoparticles (13). In this study, a nano-formulation of curcumin was synthesized using cyclodextrin, as a neutral soluble vehicle, to increase the efficiency of pulmonary drug delivery.