1. Background
Type 2 diabetes mellitus (T2DM) is a multifactorial chronic metabolic disease that accounts for more than 90% of patients with diabetes mellitus (DM). It is often characterized by insulin resistance in target organs and impaired insulin secretion (pancreatic β-cell dysfunction) caused by end pancreatic β-cell failure (1-3). According to the World Health Organization (WHO), more than 400 million people live with diabetes worldwide. DM claims 1.6 million lives annually and has been the seventh leading cause of death in 2016 (4). Approximately 70 to 97% of those with T2DM have at least one lipid abnormality, known as diabetic dyslipidemia (5).
Dyslipidemia is prevalent among those who suffer from T2DM, mainly because insulin resistance or deficiency can affect major pathways and enzymes that play a unique role in lipid metabolism. In addition, it is one of the major risk factors of atherosclerosis and coronary artery disease (CAD) in T2DM cases (6). In most cases, T2DM associated dyslipidemia is characterized by elevated triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C), as well as decreased levels of high-density lipoprotein cholesterol (HDL-C). There are evidence suggesting the impairing effect of hyperglycemia on lipoprotein lipase activity, a significant factor in the metabolism of TG-rich lipoproteins, that is associated with increased free fatty acid flux from insulin-resistant fat cells (7). Poor control of glycemic indices (GIs) results in hyperlipidemia secondary to T2DM. In such cases, effective methods of GIs and lipid profile (LP) control should be applied until achieving normal levels. Currently, these methods are recognized as the optimal treatment (6).
There is a large body of evidence regarding the anti-diabetic effects of several plant species in patients who did not respond to insulin, oral anti-diabetic drugs (OAD), and other available interventions (8). Recently, there has been significant growth in herbal products in both developing and developed countries, mainly due to their natural origin, possibly fewer side effects, and fewer adverse reactions for DM treatment (9). In addition, the WHO has recommended medicinal herbs for DM control (10). Many studies demonstrated the anti-diabetic and anti-lipidemic effects of herbal products such as Cinnamomum (11), Berberis vulgaris fruit (12), Galega officinalis leaves (13), Vaccinium bracteatum thumb leaves (VBTL) (2), and Trigonella foenum-graecum seeds (14). However, according to the best knowledge of the authors, no study has investigated their mixed effects in one capsule.
2. Objectives
The current study aimed to investigate the potential effects of a combined herbal capsule (CHC) with a mixture of powdered herbal products (including Cinnamomum, B. vulgaris fruit, G. officinalis leaves, VBTL, and T. foenum-graecum seeds) as a nutritional supplement on the levels of GIs [fasting blood sugar (FBS), two hours postprandial (2hpp), blood sugar, and glycosylated hemoglobin (HbA1c)] and LP [LDL-C, HDL-C, TG, and total cholesterol (TC)] in T2DM cases.
3. Methods
This randomized, single-blind, placebo-controlled clinical trial was conducted on 92 patients with T2DM admitted to the endocrinology and metabolism clinic of the Imam Khomeini Hospital of Khomein city (Iran) from November 2018 to March 2019. It should be noted that eligible participants were selected after applying inclusion and exclusion criteria. The aim was to investigate the potential effects of CHCs with a mixture of powdered herbal products as a nutritional supplement on GIs and LP. All participants were blinded to the received intervention and their study group. The inclusion criteria were being 18 to 60 years old; HbA1c > 7%; FBS between 126 - 200 mg/dL; confirmed diabetes diagnosed (based on the American Diabetes Association's criteria 2017) for at least six months; receiving oral anti-diabetic drugs (metformin and sulphonylurea); no change in the dose of lipid-lowering drugs over the past 8 weeks; and abnormal lipoprotein levels in patients with T2DM. The exclusion criteria included pregnancy; suffering from any malignancy; psychological and mental status disorders; cardiac failure, liver, and respiratory diseases; having nephropathy and bleeding disorders; history of receiving anticoagulant drug; taking less than 90% of the prescribed capsules; unwillingness to continue the study; and abnormal changes or significant adverse events in clinical test results.
The sample size was calculated as 40 subjects in each group, using the sample size formula, and with a 95% confidence interval, test power of 80%, and α of 0.05. To ensure the adequacy of the sample size and considering the possible dropout of participants, particularly regarding the three months follow-up, the final sample size for each group was determined to be 46 in each group, which means considering a drop of 15 percent. Before random allocation for the parallel groups, all participants were referred to the laboratory for GI and LP tests. Laboratory tests were evaluated by an endocrinologist to select eligible cases. Next, eligible participants were randomly allocated to either treatment (CHC) or control [placebo capsule (PC)] groups following a 1: 1 ratio according to the block randomization method with a block size of 4. The treatment and control groups were labeled A and B, respectively. All possible balanced combinations were assigned within a block size of four with six phases of letters (ABAB, BAAB, BBAA, AABB, BABA, ABAB). Each of the phases was written on a separate card and placed in a box. Each time, a card was randomly drawn out of the box. Afterward, letters relevant to each phase were sequentially recorded on a sheet (AABBABABBBAA…) and then placed again in the box. The procedure was repeated 23 times. One of the researchers sequentially coded the recorded letters in the 23 phases from 1 to 92 (A1A2B3B4 … B92). Every letter was placed into a small envelope and coded by numbers [code1(A), code2(A), code3(B), code4(B), … code92(B)]. An endocrinologist sequentially opened the envelopes from 1 to 92. He was unaware of the content of the envelopes (A or B).
The researcher recorded the content of each envelope only according to the original form of the randomization results. Those with an A labeled card were assigned to the treatment group and received the CHC, while those with a B label were assigned to the control group, who received a placebo. Forty-six patients were assigned to the treatment group, and 46 patients were assigned to the control group.
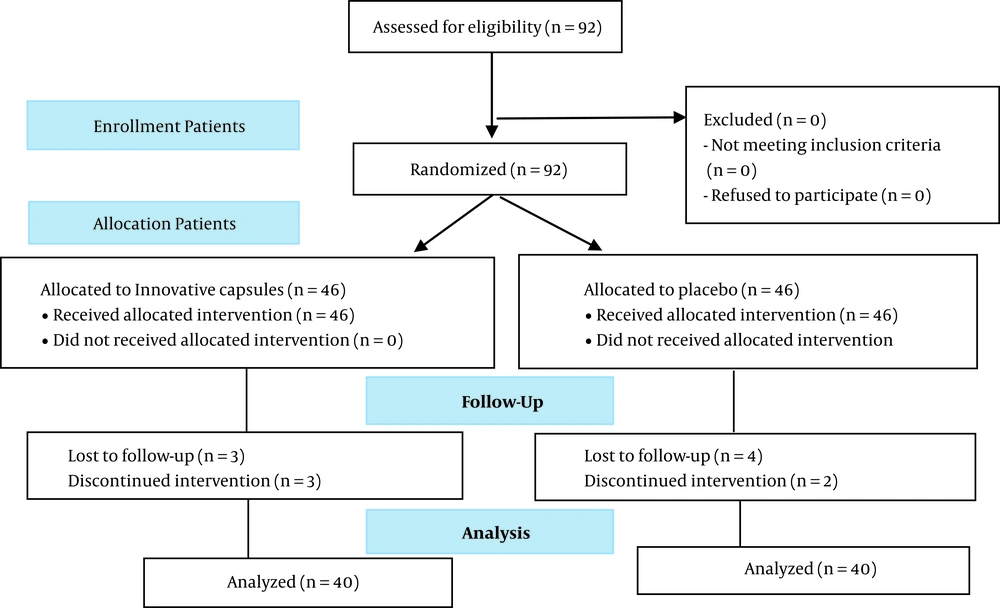
The groups’ allocation, interventions, and follow-up, and the analysis of the results are indicated in Figure 1.
3.1. Intervention
The researcher assigned 46 participants to the treatment group and 46 participants to the control group to investigate the effects of two types of capsules on GIs and LP outcomes. Patients in either group received CHC and PC twice a day (before breakfast and before bedtime at night) for three months. The CHC was similar to the placebo in terms of size, weight, texture, and shape. To reduce the attrition rate and to identify any possible side effects, all participants were reminded of the importance of taking tablets by phone calls weekly. In addition, it is worth noting that all participants were asked and trained to consume a regular diet and drug regimens. The periods of enrollment, follow-up, interventions, and assessments are presented in Table 1.
| Time Point | Study Period | |||||
|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-allocation (3 Months After the Intervention) a | Analysis | |||
| November | December | January | February | March | ||
| Enrolment | ||||||
| Eligibility screen | * | |||||
| Informed consent | * | |||||
| Allocation | - | * | ||||
| Interventions, mg | - | - | * | * | * | - |
| CHC 500 | ||||||
| PC 500 | - | - | * | * | * | - |
| Assessments | * | * | - | - | - | * |
| Medical history taking | ||||||
| GIs | * | * | - | - | - | * |
| LP | * | * | - | - | - | * |
| Capsules consumption | - | - | * | * | * | - |
| Significant side effects | * | - | * | * | * | * |
a Follow up every week by phone.
3.2. Data Collection and Outcome Measures
Data were collected using a demographic form, including age, gender, body mass index, marital status, time since diagnosis of diabetes, and education level. The validity of the form was confirmed by content validity. Data were collected through interviewing participants as well as reviewing medical records. Data on GI and LP were measured both before and 3-months after providing the intervention with the following methods. After 12h of fasting, 5 mL blood was collected from all participants and sent to the laboratory of the Imam Khomeyni Hospital to measure the FBS, HbA1C, TG, total cholesterol, LDL-C, and HDL-C concentrations. Furthermore, 2hpp was analyzed 2 hours after a standard breakfast. An auto analyzer (made in Italy), Pars Azmoon glucose kits, Bio-system kits, and the colorimetric method were used to measure HbA1C after primary separation via ion-exchange chromatography (biosystems).
3.3. Preparation of the Herbal and Placebo Capsules
In this study, CHCs and PCs were made by the Salamat Gostar Artiman Pharmaceutical Company and had a 500 mg weight.
Useful components of CHCs: (1) 100 mg powder of barberry or B. vulgaris fruit. The company purchased barberry fruits. The fruits were dried at 55°C for three days and then slightly crushed in a mortar. Finally, the obtained barberry powder was stored at 4°C in a dark room; (2) 50 mg cinnamon. The company purchased fresh Cinnamomum (cinnamon bark) from a local herbal market in Tehran, Iran. cinnamon barks were washed, dried, and ground in a mechanical grinder. The obtained powder was filtered (mesh of 50) and stored; (3) 50 mg VBTL powder. The company purchased the fresh VBTL, which were then dried and ground into a fine powder and kept in sealed plastic bags at room temperature; (4) 50 mg Galega officinalis leaves powder and 150 mg Trigonella foenum-graecum seed powder. The company purchased pure Galega officinalis leaves powder and pure Trigonella foenum-graecum seed powder. The powders were kept in a dark, airtight container.
Useful components of PCs: (1) 400 mg breadcrumbs.
Accessory component of CHCs and PCs: (1) 80 mg Avicel as fixed drug; (1) 20 mg magnesium stearate (MgSt) as drug lubricant.
3.4. Ethical Issues
Written informed consent was obtained from all participants before initiating the study. In addition, the current trial is registered by the Iranian Registry of Clinical Trials (IRCT No: 20170501033751N2, https://www.irct.ir/trial/34047). Also, the principles of the Declaration of Helsinki were followed. The study is approved and supported by the Research Ethics Committee of the Khomein University of Medical Sciences (code IR.Khomein.REC.1397.008).
3.5. Statistical Analysis
Data analysis was carried out based on the study protocol (on-treatment). Descriptive statistics were used to show the number, percentage, mean, and standard deviation (SD). Statistical analysis was administered using chi-square and paired and independent t-test by SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was applied to test for a normal distribution. Statistical significance was considered when P-value < 0.05.
4. Results
Data of 80 subjects were included in the final analysis. Demographic characteristics of participants are provided in Table 2. There was no significant difference between both groups concerning the demographic characteristics.
| Characteristics | Treatment Group (n = 40) | Control Group (n = 40) | P-Value |
|---|---|---|---|
| Age (y) | 55.15 ± 6.14 | 53.67 ± 7.72 | 0.34 b |
| BMI (kg/m2) | 27.89 ± 2.95 | 26.75 ± 3.49 | 0.11 b |
| Duration of diabetes (y) | 8.10 ± 3.32 | 6.02 ± 3.49 | 0.06 b |
| Gender | 0.63 c | ||
| Female | 12 (30) | 14 (35) | |
| Male | 28 (70) | 26 (65) | |
| Marital status | 0.35 c | ||
| Married | 38 (47.5) | 40 (50) | |
| Single | 1 (1.2) | 0 (0) | |
| Widowed | 1 (1.2) | 0 (0) | |
| Education level | 0.52 c | ||
| Illiterate | 13 (16.2) | 7 (17.5) | |
| Elementary | 15 (18.8) | 15 (18.8) | |
| Secondary school diploma | 6 (7.5) | 8 (10) | |
| Diploma | 3 (3.8) | 4 (5) | |
| Academic | 3 (8.3) | 6 (7.5) | |
| Occupation status | 0.44 c | ||
| Employee | 2 (2.5) | 0 (0) | |
| Pensionary | 7 (8.8) | 9 (11.2) | |
| Housewife | 24 (30) | 21 (26.2) | |
| Unemployment | 5 (6.2) | 5 (6.2) | |
| Self-employment | 2 (2.5) | 5 (6.2) |
Abbreviations: SD, standard deviation; BMI, the body mass index.
a Values are expressed as mean ± SD or No. (%).
b Chi-square test for categorical variables.
ct-test for quantitative variables.
The mean GIs (FBS, 2hpp, and HbA1c) levels were significantly different among patients in the treatment group after the intervention (P < 0.05). While in the control group, the mean GIs levels were not significantly different after the intervention (P > 0.05) (Table 3). The results showed that the mean TG, LDL-C, and HDL-C levels were significantly improved in the treatment group after the intervention (P < 0.05), but the TC level remained unchanged (P > 0.05). The findings also revealed that the mean LP level did not change after the 3-month in the control group (P > 0.05) (Table 4). The mean GIs and LP levels (TG, LDL-C, except for the TC) were improved after the 3-month intervention in the treatment group compared to the control group. However, no significant difference was observed between the study groups. Moreover, the mean HDL-C level was significantly improved in the treatment group compared to the control group after the intervention. Interestingly, no patients reported any significant side effects during the study period.
| Characteristics | Steps, Mean ± SD | Mean Difference | P-Value* | |
|---|---|---|---|---|
| Before the Intervention | After the Intervention | |||
| FBS (mg/dL) | ||||
| Control group (n = 40) | 193.16 ± 56.67 | 195.63 ± 57.21 | 2.47 (-22.9 to 27.8) | 0.805 a |
| Treatment group (n = 40) | 215.41 ± 62/02 | 173.81 ± 57.32 | -41.60 (-68.2 to -15.0) | 0.002 a |
| P-value b | 0.139 b | 0.133 b | ||
| 2hpp (mg/dl) | ||||
| Control group (n = 40) | 259.13 ± 82.11 | 246.29 ± 87.68 | -12.84 (-26.9 to 1.2) | 0.071 a |
| Treatment group (n = 40) | 270.0 ± 81.91 | 224.41 ± 83.54 | -45.59 (-86.5 to -4.6) | 0.03 a |
| P-value b | 0.695 b | 0.864 b | ||
| HbA1c (%) | ||||
| Control group (n = 40) | 8.58 ± 1.38 | 8.38 ± 1.65 | -0.20 (-2.0 to 1.6) | 0.99 a |
| Treatment group (n = 40) | 8.52±1.32 | 8.10 ± 1.24 | -0.42 (-0.75 to -0.09) | 0.01 a |
| P-value b | 0.857 b | 0.258 b | ||
Abbreviations: FBS, fasting blood sugar; 2hpp, two hours postprandial; HbA1c, glycosylated hemoglobin.
a Paired t-test.
b Independent Student’s t-test.
| Characteristics | Steps, Mean ± SD | Mean Difference | P-Value a | |
|---|---|---|---|---|
| Before the Intervention | After the Intervention | |||
| TG (mg/dL) | ||||
| Control group (n = 40) | 188.79 ± 3.8 | 175.78 ± 6.8 | -13.01 (-29.5 to 3.5) | 0.11 a |
| Treatment group (n = 40) | 169.8 ± 94.03 | 151.8 ± 94.9 | -18.0 (-35.8 to -0.2) | 0.03 a |
| P-value b | 0.345 b | 0.226 b | ||
| LDL-C (mg/dL) | ||||
| Control group (n = 40) | 94.25 ± 5.6 | 90.25 ± 2.2 | -4.0 (-9.3 to 1.3) | 0.13 a |
| Treatment group (n = 40) | 98.2 ± 34.2 | 90.4 ± 35.6 | -7.8 (-13.1 to -2.4) | 0.005 a |
| P-value b | 0.589 b | 0.983 b | ||
| HDL-C (mg/dL) | ||||
| Control group (n = 40) | 42.7 ± 6.4 | 41.8 ± 9.6 | -0.90 (-4.5 to 2.7) | 0.53 a |
| Treatment group (n = 40) | 47.5 ± 11.5 | 51.07 ± 12.8 | 3.57 (0.68 to 6.5) | 0.01 a |
| P-value b | 0/026 b | < 0.001 b | ||
| Total cholesterol (mg/dL) | ||||
| Control group (n = 40) | 169.34 ± 4.8 | 161.34 ± 6.6 | -8.0 (-16.9 to 0.9) | 0.08 a |
| Treatment group (n = 40) | 169.7 ± 56.6 | 162.4 ± 59.4 | -7.3 (-17.5 to 2.9) | 0.15 a |
| P-value b | 0.974 b | 0.943 b | ||
Abbreviations: TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
a Paired t-test.
b Independent Student’s t-test.
5. Discussion
As mentioned before, this is the first study that has investigated the potential effects of CHC combined with powdered herbal products, as a nutritional supplement, on GIs and LP in those suffering from T2DM. Our literature review revealed that previous studies only explored the effect of five herbal products (e.g., “Cinnamomum” and “G. officinalis") on GIs and LP levels in those with T2DM. It was assumed that CHC and oral hypoglycemic agents can better improve glycemic and lipemic control compared to the PC and oral hypoglycemic agents. We also hypothesized that CHC with a mixture of powdered herbal products (in one capsule as a nutritional supplement) would better improve GIs and lipemic levels compared to the sole administration of these herbs. Before discussing the effects of CHCs on GIs and LP levels, each component of CHCs should be explained by reviewing previous studies.
barberry or B. vulgaris is a herb of the Berberidaceae family that widely grows in Asia and Europe (15). Initially, the effect of barberry on lowering glucose was observed during its administration for diarrhea treatment in diabetic patients (16). Previous studies reported its impact on controlling depression, hyperlipidemia, liver disease, and hyperglycemia (17, 18). Barberry has also been identified as an effective agent against cholesterol, possibly via decreasing the PCSK9 expression. It also can decrease LDL receptor degradation, increases the uptake of plasma cholesterol by the liver, and improves the blood's LDL‐C clearance and its transformation to the bile. It also can decrease the intestinal absorption of cholesterol and increases fecal excretion. It is associated with improved hepatic turnover of cholesterol, the production of bile acids, and the stimulation of AMP‐activated protein kinase, which in turn can restraint the synthesis of fatty acids (19). Lan et al., in their meta‐analysis, showed that berberine not only could reduce TG, TC, and LDL‐C levels but also increased the HDL‐C content (20). Safari et al. reported that although barberry supplementation could significantly improve insulin levels, it might not impact other GIs (15). Nevertheless, previous clinical trials could not reach a definite conclusion concerning the effect of barberry on GIs. T. foenum-graecum leaves (fenugreek) are a traditional promising medicinal herb used to treat diabetes, obesity, and other diseases. The herb belongs to the leguminous family, extensively cultured in many countries, including Morocco, Egypt, China, India, Ethiopia, Turkey, Ukraine, and Greece (21). It contains active components such as flavonoids, alkaloids, saponins, and steroids. In addition, it is rich in soluble fiber, which can decrease blood sugar by hindering the assimilation and retention of carbohydrates (22). Moreover, it has antioxidant, antihyperlipidemic, anti-inflammatory, antibacterial, antifungal, and galactagogic properties (23). Several clinical trials revealed that T. foenum-graecum leaves could promote most metabolic symptoms associated with type 1 and type 2 diabetes in humans by bringing down blood glucose levels and improving glucose tolerance (24). G. officinalis, commonly known as goat’s rue, is a natural herb that grows in southeastern parts of Europe and the Middle East. As previously described, G. officinalis is used in traditional phytotherapy due to its diuretic and hypoglycemic properties and weight-reducing ability (25). Methanolic extracts of phytotherapy could significantly improve LP in a clinical study.
As an essential member of biguanide drugs, metformin production is based on guanidine derivatives isolated from G. offcinalis. Indeed, the only example of an approved anti-diabetic or anti-hyperglycemic drug extending from a herbal source with a long history of use for diabetes is metformin isolated from G. officinalis (13). Abtahi-Evari et al. reported that G. officinalis was beneficial for the treatment of diabetes through improving tissue sensitivity to insulin and preventing tissue damages (26). Moreover, Shojaee et al. concluded that G. officinalis extracts contain compounds with the potential of hypoglycemia and weight-reducing properties that can be used as a herbal treatment for T2DM and weight gain (13). VBTL (called Wufanshu in China) belongs to the same genus as blueberry and grows in East Asia, especially in the east and south of China. It has several physiological functions, including anti-fatigue, digestion resistibility, antioxidant activity, and tyrosinase inhibiting properties (27, 28). Wang et al. showed that aqueous and ethanolic VBTL extracts showed a potential hypoglycemic effect on streptozotocin-induced diabetic mice. They also showed that VBTL could significantly decrease the LP levels (2, 8).
It has been indicated that cinnamon (Cinnamomum spp), as a dietary component, contains biologically active substances that can regulate blood glucose by insulin-like properties. Such properties increase glucose uptake by activating the insulin receptor's autophosphorylation, glycogen synthase activity, and insulin receptor kinase activity (3). Cinnamon is a prophylactic supplement of insulin resistance, metabolic syndrome, T2DM, hyperlipidemia, and arthritis in the market (29). More than 250 species of genus Cinnamomum (Lauraceae) are identified, and C. cassia and C. zeylanicum are the main varieties with applications in medicine (3). On the other hand, Zare et al. reported no significant effect on HbA1c levels (30). Also, meta-analyses conducted by Allen et al. (31) and Alanazi and Khan (32) suggested a significant improvement in FBS levels and LP parameters. Ranasinghe et al. (3) were optimistic about the effect of Cinnamomum on decreasing GIs. According to the evidence in previous studies regarding the mentioned herbs, this reduction was not beyond our expectations. Combined use of herbs in a single capsule could reduce GIs and LP levels after 3 months. Our findings indicated a significant improvement in overall GIs and LP levels, except for TC in the treatment group after the intervention. The mean GIs and LP levels (TG and LDL-C except for TC) improved after the 3-month intervention in the treatment group compared to the control group, but the difference was not statistically significant. Moreover, the mean HDL-C level significantly improved after the 3-month intervention in the treatment group compared to the control group. Based on the findings of the present study, CHCs, as a nutritional supplement, may have positive effects on GIs and LP in patients with T2DM. In addition, CHCs may have different mechanisms for influencing GIs and LP levels (12, 30). We cannot further discuss the effects of CHCs because this was the first time that a mixture of powdered herbal products has been used as a nutritional supplement, which means no similar study to make a comparison.
5.1. Conclusion
This study demonstrated that CHCs can improve GIs LP levels (TG, LDL-C, and HDL-C, except for TC), which indicates their potential to be used for T2DM management. Moreover, we did not record any significant side effects caused by CHCs used in this study. It appears that using CHCs as adjuvant therapy with conventional hypoglycemic and lipid-lowering drugs, as well as modifying the lifestyle could remarkably improve glycemic control and restrict DM-associated complications. Nevertheless, future studies are needed to extend our knowledge beyond the effects of CHCs on T2DM control as a formal nutritional supplement.
5.2. Limitations
It is necessary to mention some limitations and biases of our study, including the withdrawal of some participants due to the long duration of the intervention and the non-generalizability of the findings. Further studies are warranted to make a definitive conclusion regarding the effects of CHCs on the GIs, LP levels, and other serum biochemistry profiles with larger sample size.