1. Background
According to statistics, 2.8% of the world population is suffering from diabetes, and this number is expected to reach 5.4% by 2025. Diabetes requires early diagnosis, treatment, and lifestyle changes. Diabetes is a disease that has affected several people in the 21st century and is recognized as the fifth leading cause of death (1).
According to the data released in March 2013 by the World Health Organization (WHO), there have been 153 million registered diabetic patients since 1980. By 2008, the number of these patients has increased, with 347 million people being diagnosed with diabetes worldwide. It is also believed that the number of patients with diabetes will increase to 380 million by 2025, representing 7.1% of the world's adult population (2).
Glucosidases are a group of enzymes that play a vital role in carbohydrate digestion. Glucosidase inhibitors can reduce carbohydrate digestion and prevent type 2 diabetes (3). Plants have always been very good sources of medicines, and many of the currently available drugs are extracted directly or indirectly from plants. Ethnobotanical data suggest that as many as 800 plant species may have anti-diabetic potential (4).
Salvia in Latin is called “Salvare”, which means “healing” (5). Lamiacea is one of the most well-known families of plants. Many of its variants contain essential oils with potential therapeutic activities. Salvia has 900 species, among which 58 species are found in temperate and subtropical regions of Iran, and 17 of them are endemic (6). The names of more than 400 herbal hypoglycemic species are mentioned in books, yet finding new anti-diabetic drugs is still attractive (7).
2. Objectives
In this study, we investigated different species of Salvia in terms of inhibitory effects on alpha-glucosidase as an approach to the treatment of diabetes.
3. Methods
p-nitrophenyl-PD-glucopyranoside (PNPG) (code: N1377) and the alpha-glucosidase enzyme of Saccharomyces cerevisiae (code: G0660-750UN) were purchased from Sigma Aldrich, USA, and other chemicals were bought from Merck Co.
3.1. Specimen Collection and Preparation
S. officinalis and S. macilenta plants were collected by Mr. Asadipour in March 2018, from around Bandar Abbas, which were identified and approved by experts and then extracted. The voucher number was deposited in the Medicinal Plants Processing Research Center, School of Pharmacy, Shiraz University of Medical Sciences. The voucher numbers of S. officinalis and S. macilenta are MPPRC-90-18, and MPPRC-93-1, respectively. Initially, the leaves of all plants were dried, chopped, and prepared with dichloromethane extracts and were concentrated using a rotary evaporator. Then, the methanol solvent was added to the residue, and the extracts were again concentrated using a rotary evaporator. The required extracts were thus prepared and stored at 4°C.
The inhibition of alpha-glucosidase (EC: 3.2.1.20) was evaluated with minor modifications by concentrations of 1, 2, 5, 10, and 20 mg/mL of dichloromethane and methanol extracts of S. macilenta and S. officinalis according to the method reported by McCue et al. (8). In this method, PNPG (4-nitrophenyl α-D-glucopyranoside) is used as a substrate that is hydrolyzed to para-nitrophenolate product (yellow) and glucose by the alpha-glucosidase enzyme. In addition, enzyme activity is determined by measuring the amount of produced para-nitrophenol absorption. If the plant extract has an alpha-glucosidase inhibitory effect, there is lower uptake than that in the control (no inhibitor reaction). Acarbose was employed as the standard in this study (8).
We primarily added 0.5 mL of DMSO solvent and ethanol to the samples containing 10 mg of the extracts in 2 mL microtubes. Subsequently, we prepared different concentrations of the plant extracts. Then, we prepared similar concentrations of the extracts for acarbose.
3.2. Preparation of Alpha-Glucosidase and PNPG
The method reported by McCue et al. (8) was employed with a few modifications for making a 5 cc alpha-glucosidase solution. First, we poured 1 mg of alpha-glucosidase (25 u/mL) into a 2 mL microtube. Afterward, we increased it to a 5 cc volume by 0.1 M of sodium phosphate buffer (pH = 6.8 - 6.9). p-nitrophenyl-a-D-glucopyranoside (11 mM) was dissolved in the above-mentioned buffer (pH 6.9) and used as the substrate solution.
3.3. Investigating the Inhibitory Effects of Salvia Extracts and Acarbose on Alpha-Glucosidase
Starting the experiment in a row of ELISA microplate strips, then, 125 λ phosphate buffers of 0.1 M were added to all wells. Two blank wells received 5 λ buffers, and the same amount of alpha-glucosidase was added to other wells. Subsequently, 20 λ of the inhibitor sample was added to the sample blanks and sample wells.
The same amounts of DMSO and ethanol were added to the control blanks and two control wells, respectively. The microplate was then incubated in a refrigerated shaking incubator (manufactured by the Hanyan Scientific Corporation of Korea) at 37°C for 15 minutes. The microplate was then removed, and 20 μL of 0.033 mg/10 mL para-nitrophenol glucose solution was added to all wells. The microplate was re-incubated at 37 °C for 15 min. The decomposition of PNPG to glucose and yellow nitrophenolate resulted in a yellowish-green solution color. If the plant extracts had inhibitory effects, the amount of produced dye decreased with the increase of the inhibition.
The microplate was then removed, and 80 λ of sodium carbonate 0.2 M was added as a stopper to all wells. Afterward, at 450 nm, the absorbance was determined with a Microplate Reader (multi-mode reader-synergy HTX manufactured by Biotek, USA). The percentage of inhibition was then calculated based on the following formula: %I = [(OD control - OD blank control) - (OD sample - OD blank sample)]/[(OD control - OD blank control) × 100].
Due to the high sensitivity of alpha-glucosidase and the need to maintain the solutions at 4°C, the experiments were carried out on an ice pack. Following each experiment, we placed the PNPG solution in the refrigerator at -17°C.
3.4. Alpha-Glucosidase Enzyme Kinetics
Different concentrations of substrate were prepared. The extract concentration, which caused 50% of the inhibition, was used. The experiment was continued until the Michaelis Menten curve was obtained. The amount of adsorption was then converted into activity by using the molar extinction coefficient of the reaction product. Afterward, Km and Vmax were calculated using GraphPad Prism software.
3.5. Statistical Analysis
The mean and standard deviation (SD) were calculated utilizing GraphPad Instat 3 and one-way analysis of variance (ANOVA) with Tukey posttest. If P < 0.05, the difference was considered to be significant. GraphPad Prism 7 software was employed for the calculation of Km and Vmax and the determination of inhibitor type. For this purpose, we primarily converted the amount of reading absorption into enzyme activity using the product (paranitrophenolate and glucose) molar extinction coefficient. Afterward, the values of Km and Vmax were calculated using Graphpad Prism software.
4. Results
The extracts had significant inhibitory effects on alpha-glucosidase inhibition. Among the studied extracts, S. macilenta had more efficient inhibitory effects.
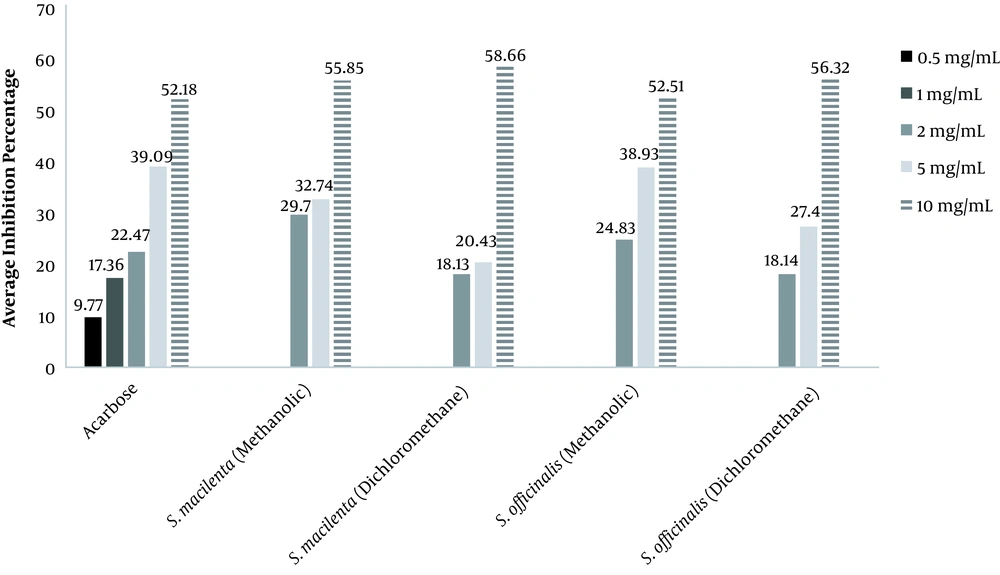
Figure 1 represents the percentage of alpha-glucosidase inhibition by plant extracts and acarbose.
Table 1 demonstrates the IC50 ± SD value after three replicate trials. As can be seen, the IC50 of acarbose is very close to that of plant extracts.
| Acarbose | S. Officinalis (Methanol) | S. Officinalis (Dichloromethane) | S. Macilenta (Methanol) | S. Macilenta (Dichloromethane) |
|---|---|---|---|---|
| 8.82 ± 0.15 | 8.99 ± 0.85 | 8.95 ± 0.23 | 8.73 ± 0.26 | 8.96 ± 0.1 |
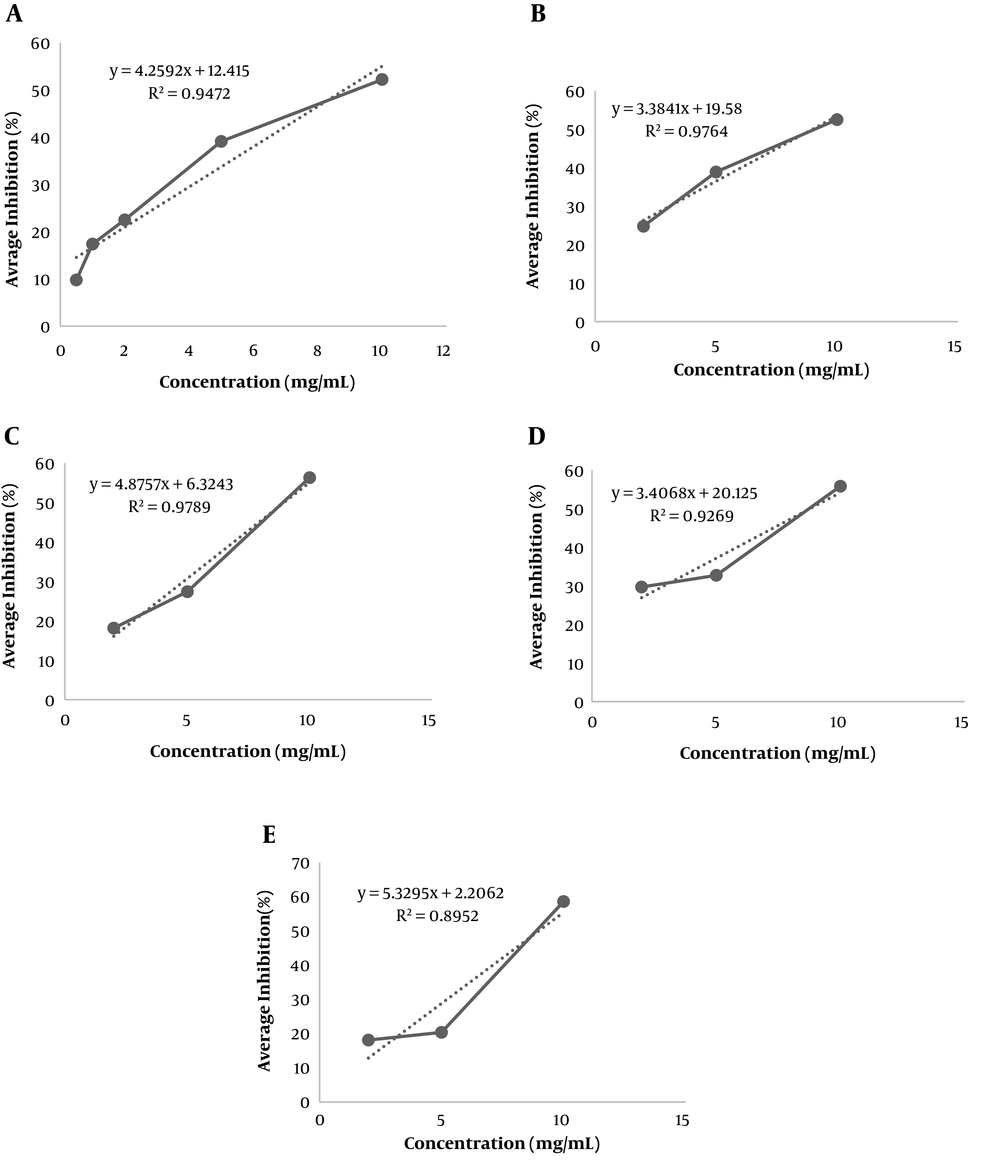
Figure 2 depicts the concentration versus the percentages of alpha-glucosidase inhibition by the studied extracts and acarbose.
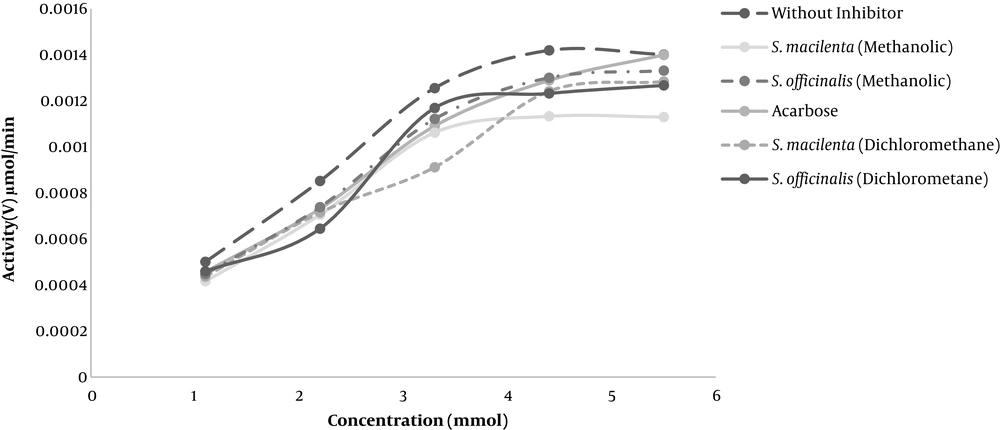
The saturation diagram of alpha-glucosidase is shown in Figure 3. The comparison of Km and Vmax in the presence and absence of inhibitors indicated the inhibition type. If Vmax is constant and Km increases, the inhibition is competitive, and if Vmax is decreased and Km is constant, the inhibition is noncompetitive. If Vmax and Km are reduced, the inhibition is uncompetitive; otherwise, the inhibition is mixed.
Accordingly, the activity without the inhibitor was higher than that of the Salvia species studied.
Table 2 illustrates the values of Km ± SD and Vmax ± SD. Based on this table, a comparison of Km and Vmax with and without inhibitors implied that the acarbose inhibition type was competitive and the inhibition type in methanol extract of S. macilenta, and dichloromethane extract of S. officinalis were uncompetitive. Other extracts showed mixed inhibition.
| Extract | Km ± SD (mmol) | Vmax ± SD (µmol/min) | Inhibition Type |
|---|---|---|---|
| Acarbose (standard) | 5.22 ± 1.04↑ | 0.0028 ± 0.0003(constant) | Competitive |
| S. officinalis (methanol) | 4.37 ± 0.57↓ | 0.0025 ± 0.0001↓ | Un competitive |
| S. officinalis (dichloromethane) | 4.46 ± 1.17↓ | 0.0024 ± 0.0003↓ | Un competitive |
| S. macilenta (methanol) | 3.73 ± 0.83↓ | 0.002 ± 0.0002↓ | Un competitive |
| S. macilenta (dichloromethane) | 5.36 ± 0.65↑ | 0.0025 ± 0.0001↓ | Mixed |
| Without inhibition | 4.47 ± 1.008 | 0.0028 ± 0.0003 | - |
The inhibition type of the studied extracts was not affected by the substrate concentration and had a greater enzyme inhibitory capacity than acarbose as competitive inhibitors.
5. Discussion
Diabetes mellitus is characterized by an absolute or relative deficiency in insulin secreted by beta-pancreatic cells and an increase in insulin resistance or impaired insulin action in target tissues (9, 10).
Alpha-glucosidase hydrolyzes α (1-4) bonding from the non-regenerating end of starch and produces glucose. The alpha-glucosidase enzyme comprises five domains (A, B, C, D, and E) and 868 amino acids. The catalytic site of alpha-glucosidase contains Asp542-Glu444-Asp443 amino acids (11).
There are approximately 945 species of Salvia, highly diverse in ecology, life form, morphology, and karyology (12). It has been reported that plant foods, rich in polyphenols, have similar effects with insulin on glucose utilization. Moreover, inhibitors of key enzymes, such as alpha-amylase and alpha-glucosidase, prevent type 2 diabetes (13).
The most dominant metabolites in the Salvia species are terpenoids, which have the highest biological activity in these species (14, 15). A wide range of natural compounds such as flavonoids, steroids, terpenoids, for instance, have been identified as alpha-glucosidase inhibitors (16).
The results of this study shed light on the fact that dichloromethane and methanol extracts of S. officinalis and S. macilenta affect alpha-glucosidase inhibition and their IC50 (Table 1).
These results are in line with the results of a study by Hamidpour (2014) reporting that S. officinalis is utilized as a traditional anti-diabetic drug in several countries, and its reducing effects on glucose were shown in animal studies. The study found that methanol extract of S. officinalis significantly reduced serum glucose in type 1 diabetic rats without affecting pancreatic insulin production. In addition, it has been suggested that consumption of S. officinalis tea is as effective as metformin in reducing blood sugar, and by lowering glucose production in the liver and increasing insulin function, it is capable of lowering blood sugar (17).
These results are consistent with the findings of Ghorbani (2016), who identified the phytochemical compounds of S. officinalis in flowers, leaves, and stems, involving alkaloids, carbohydrates, fatty acids, glycoside derivatives such as flavonoid glycosides, phenolic compounds, and terpenoids which had anti-mutagenic, anti-inflammatory, and hypoglycemic effects and could increase memory (18).
The findings of Proenca (2017) revealed that the flavonoids present in the genus Salvia could be substituted for other alpha glucose inhibitors, and their buildings are the determining factors for the above-mentioned effects. Proenca used acarbose as a positive control (19).
Diabetes mellitus is believed to be the most serious chronic metabolic disease. According to the WHO, nearly 180 million people are currently diagnosed with type 2 diabetes. In addition, serious complications of diabetes mellitus, such as retinopathy and cataracts, could also trigger serious problems for patients. One of the treatments for diabetes is to delay glucose uptake by inhibiting carbohydrate-hydrolyzing enzymes (20, 21). Studies have shown that flavonoids are a good alternative to alpha-glucosidase inhibitors. The structure of the flavonoids and the position and number of OH are the determining factors (19). Research has shown that about 411 compounds of terpenes and flavonoids, phenols, and steroids have potent inhibitory activity against alpha-glucosidase (22).
The percentage of inhibition exhibited that acarbose, as a standard at 10 mg/mL, was at the highest level of inhibition with 52.18%. At the concentration of 5 mg/mL, methanol extract of S. officinalis depicted the highest inhibition (38.9%) after acarbose. Based on Table 2, the inhibition type showed that methanol extract of S. macilenta with Km = 3.73 ± 0.83mmol and Vmax = 0.002 ± 0.0002μmol/min was uncompetitive. Dichloromethane extract of S. macilenta with Km = 3.36 ± 0.65 mmol and Vmax = 0.0025 ± 0.0001 μmol/min revealed mixed inhibition which is not affected by the substrate concentration. Acarbose with Km = 5.22 ±1.04 mmol and Vmax = 0.0028 ± 0.0003 μmol/min revealed competitive inhibition which is affected by the substrate concentration. Dichloromethane and methanol extracts of S. officinalis, based on Table 2, showed mixed inhibition with Km = 4.46 ± 1.17 mmol, Vmax = 0.0024 ± 0.003 μmol/min, and Km = 4.37 ± 0.57 mmol, Vmax = 0.0025 ± 0.0001 μmol/min, respectively.
Mixed and uncompetitive inhibitors have a greater enzyme inhibitory capacity than acarbose, as a competitive inhibitor, and against acarbose, their inhibitory potency is not influenced by substrate concentration (23, 24).
One of the limitations of this study was that the experiments were only performed in vitro.
Another limitation herein was the toxic and carcinogenic effects of some plant-derived compounds, which make them unsuitable for therapeutic applications. Therefore, it is important to investigate the cytotoxic potential of plant extracts to validate the safety of medicinal plant extracts or compounds before their therapeutic applications.
Further studies should be directed towards chemical isolation, purification, and characterization of these two extracts to elucidate the compounds responsible for inhibition potential since these might play a significant role in the development of anti-diabetic agents. Furthermore, it is recommended to perform the experiments in vivo for finding the mechanisms of the utilized extracts in inhibiting alpha-glucosidase.