1. Background
Diabetes mellitus (DM) is a chronic disease, which is considered a growing health concern and affects nearly 470 million individuals worldwide (1). The underlying causes of the disease may include a range of problems such as autoimmune reactions, impaired antioxidant defense, disrupted anti-inflammatory responses, and increased oxidative stress (2). It seems that DM-caused complications such as retinopathy, neuropathy, and nephropathy are mainly affected by excessive production of reactive oxygen species (ROS) and oxidative stress.
Several plant-derived medicines have been suggested for the treatment of DM, according to different ethnomedicines (3, 4). For example, Trigonella foenumgraecum, Allium sativum, Allium cepa, and Curcuma longa showed anti-diabetic potentials in previous investigations, which may be associated with their antioxidant activities (5-7). Salvia officinalis (SVO) is a plant from Lamiaceae family, and it was reported that SVO has anti-inflammatory, anti-bacterial, and anti-mutagenic, and anti-hyperglycemic effects (8-12). Previous studies have evaluated the anti-diabetic effect of SVO, which was found to be promising (12, 13). This could be because of the previously confirmed anti-oxidative potentials of SVO, which regulate many enzymes such as superoxide dismutase, catalase, and glutathione peroxidase (14-16).
2. Objectives
The present study was designed to determine the protective effects of SVO extract administration in streptozotocin (STZ)-induced pancreatic injury in rat models using quantitative stereological methods.
3. Methods
3.1. Preparing the Extract
Salvia officinalis leaves were collected from Kohgiluyeh and Boyer-Ahmad province, south-west of Iran, in Summer, and it was authenticated (Voucher number: 157) at the research department of Agriculture and Natural Resources Engineering Organization of Yasuj, Kohgiluyeh and Boyer-Ahmad, Iran. The leaves were dried at 50°C and ground into powder. The powder (120 g) was being mixed and extracted with 600 mL ethanol aqueous solution (80:20) in a Soxhlet apparatus for 72 h. At the end of the extraction process, the solvent was filtered and evaporated by a rotary evaporator. The yield of the extract was calculated at 16%. The obtained SVO alcoholic extract was stored at -18°C until usage.
3.2. Subjects and Experimental Design
Forty-eight male normoglycemic Sprague-Dawley rats (weighted 230 ± 20 g) were kept in cages with free access to adequate water and food, and a temperature of 24 ± 2°C, as well as 12 h dark/light cycles. We randomly divided the rats into four groups (n = 12). The first group consisted of non-diabetic normal rats (C1) that were solely received 1 cc per oral distilled water daily with no other treatment. The second control group consisted of non-treated STZ-received diabetic rats (C2) receiving 1 cc per oral distilled water daily. The first experimental group (E1) received 200 mg/kg of the bodyweight of the SVO extract dissolved in distilled water via gastric intubation daily; the second experimental group (E2) also received 400 mg/kg of the SVO extract orally dissolved in distilled water daily. These doses were chosen according to a previously published article on the anti-diabetic effects of SVO extract (17).
All groups received a single dose of STZ on day 7, except for C1. We administered STZ on day 7 to let the animals spend seven days receiving the treatments as a part of their routine. The goal of this was to show the preventive effect of our treatments and to show that if these treatments are used in diet, they can or cannot lead to reduction of the chance of developing diabetes. Furthermore, the designed treatments were administered for the rats (except for C1 group that receives only distilled water) every day from day 1 to day 14.
3.3. Streptozotocin Administration and Detection of Hyperglycemia
Streptozotocin is a toxic agent that disrupts the function of insulin-producing beta cells, and it has been used in previous studies to induce DM in animal models (18-21). After an overnight fasting period, rats were injected intraperitoneally with a single dosage of 60 mg/kg body weight of STZ (Sigma-Aldrich®, Steinhelm, Germany). The blood sugar (BS) levels of the rats were tested by glucometer (Rightest GM110, Bionime™; Switzerland) every day during the injection of STZ (day 7 to day 14). Animal models, which showed a BS level of ≥ 300 mg/dL were labeled as diabetic (20, 21).
3.4. Pancreas Volume and Shrinkage Estimation
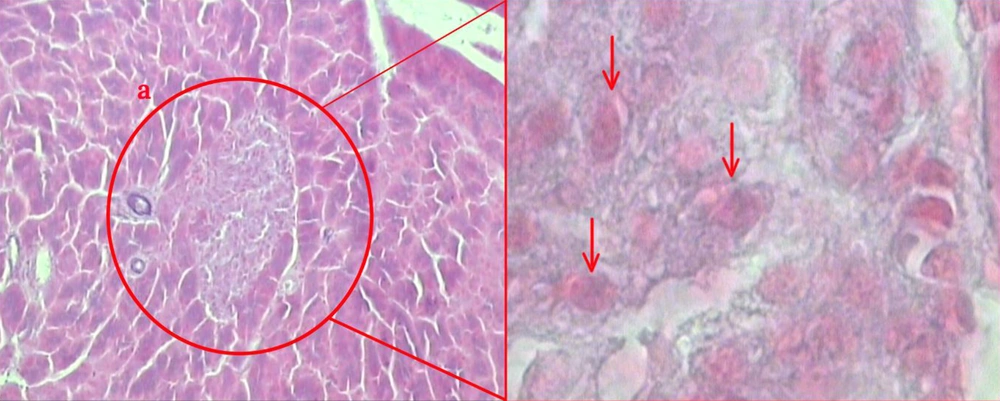
After sacrificing the animal models at the end of the investigation, rats’ pancreases were dissected by surgical procedures. Fatty and connective tissues were cleaned from removed pancreases, and primary volume (V primary) of the pancreases was measured. The samples were embedded in a paraffin block, 5 µm and 12µm sections were obtained and stained with Gomori's Aldehyde Fuchsin stain (Figure 1) (22). During the tissue processing, the final volume of the pancreases was also measured according to Cavalieri method (23). Moreover, isotropic uniform randomized (IUR) sectioning of the specimens was used to estimate shrinkage volume according to orientator method (24). The degree of shrinkage [d(sh)] was estimated using the following equation:
As the BP is that before the processing and the AP is the area of each sections after the processing.
3.5. The Pancreatic Islets’ Volume Density
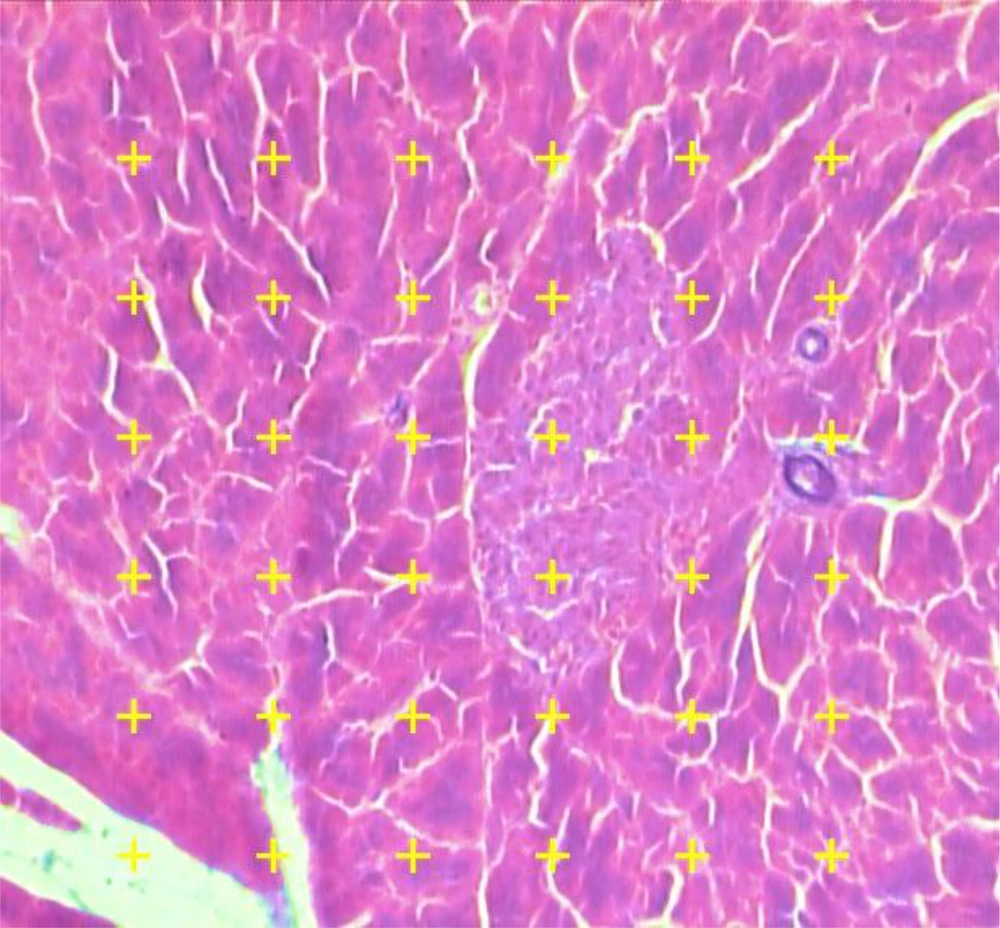
Microscopic analysis of the samples was done by a video-microscopy framework, consisting of a microscope (E-200, Nikon™, Japan) connected to a visual camera and a monitor. The volume densities (Vv) of the islets were evaluated at the magnification of 340× by using the 5 µm thickness sections and the point counting stereological method (Figure 2). The formula of measuring the Vv of the islets in this study was as the following:
As P (reference) was the number of points hitting on the reference space and P (islet) was the number of points hitting the profiles of the islets.
The pancreatic islets’ volume density (Vv) was measured using point-counting technique by means of a video microscopy system. A systematic random method was applied to select microscopic field of each slide, then, at that point, being investigated. Total amounts of points touching the islets are counted and divided by total amounts of points touching the whole tissue. The marks are counted in the case that the right upper corner of the mark touches the tissue (modified Aldehyde Fuchsin) (340×).
3.6. Numerical Density and Volume of the Beta Cells
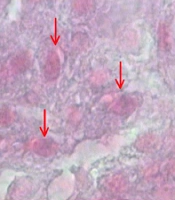
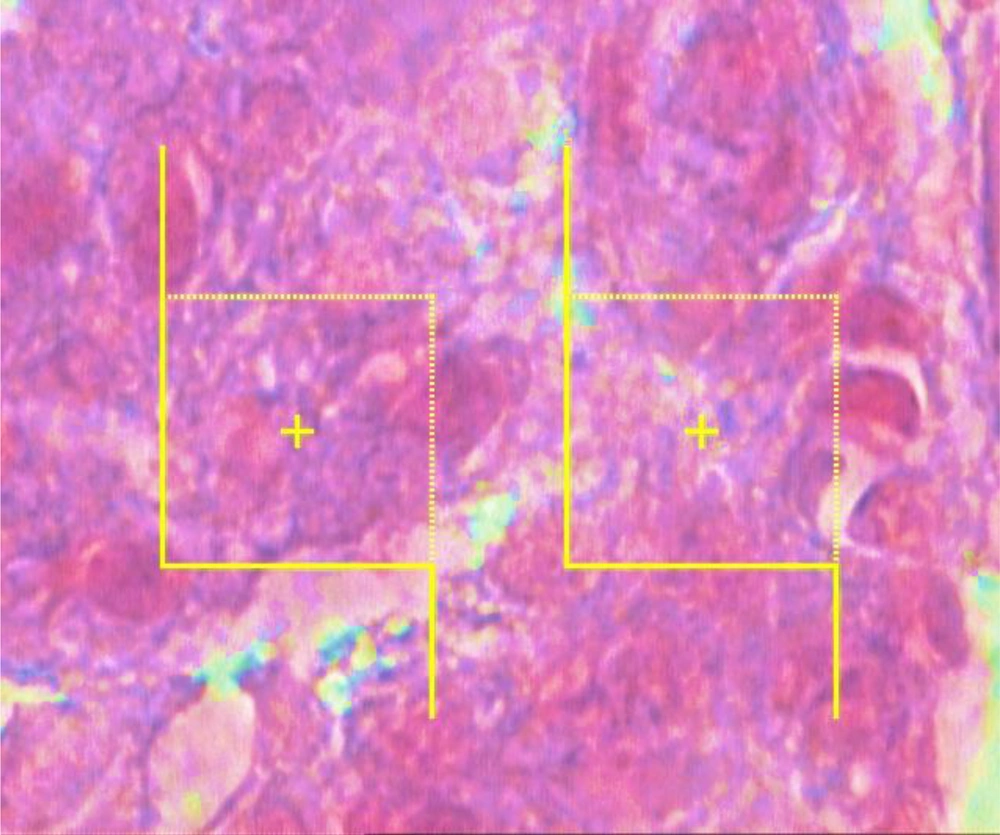
The numerical densities of the beta cells were estimated by employing final magnification of 3400× at 12 µm thickness using the “optical dissector” technique (Figure 3). The cell count was performed as per a previously confirmed technique introduced by Noorafshan et al. (25). The mean volume of the beta cells was also assessed with point-sampled intercept method (25).
To calculate the beta cells' numerical density (Nv), each cell that was within the counting box or contacted the containing borders (red dashed lines) as well as did not adjoin the exclusion margins (red solid lines) and came into maximal focus within the next traveling 5 µm optical area (height of dissector) was counted (modified Aldehyde Fuchsin) (3400×).
3.7. Ethical Issues
All national and institutional regulations considering the use of laboratory animals complied. Furthermore, the design and protocol of investigation were also affirmed by the Medical Ethics Department of Shiraz University of Medical Sciences, Shiraz, Iran (Reg. No: 90-01-61-3948).
3.8. Statistics
The derived data were analyzed and expressed as mean ± standard deviation (SD). Statistical calculations were done by application of Kruskal-Wallis and Mann-Whitney U tests to compare the rat groups as the derived data were non-parametric. All tests were done by an expert statistician using SPSS software (21.0, IBM™, USA) program. P-value ≤0.05 was regarded as statistically significant.
4. Results
Volume densities and total number of islets and beta cells in E1 and E2 groups were significantly higher than C1, and lower than C2 (Table 1). Volume densities of islets and beta cells, and total number of beta cells in E1 were significantly higher than C2 group by 69%, 274%, and 188%, respectively (P < 0.05). Volume densities of islets and beta cells in E2 group significantly increased by 152% and 187% in comparison to C2 (P < 0.005). BS levels also seemed to be lower in E1 (526 mg/dL) and E2 (479 mg/dL) groups compared to the C2 (721 mg/dL).
| Groups | Weight | Pancreas Volume | Islets | Beta Cells | Blood Sugar | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body | Pancreas | Primary | Final | Shrinkage | Vv | Total Volume | Nv | Total Number | ||
| C1 | 221.87 ± 9.16 | 860.41 ± 74.22 | 830.31 ± 45.79 | 711.79 ± 33.7 | 14.27 ± 0.15 | 0.96 ± 0.91 | 6.81 ± 2.06 | 1933.38 ± 391.27 b | 1.16 ± 0.39 b | 138.52 ± 41.23 |
| C2 | 209.43 ± 11.60 | 872.39 ± 97.24 | 918.21 ± 82.13 | 715.44 ± 92.84 | 22.35 ± 0.21c | 0.39 ± 0.73 c | 2.78 ± 0.54 c | 127.81 ± 84.57 c | 0.27 ± 0.18 c | 721.33 ± 29.31c |
| E1 | 202.37 ± 7.72 | 857.51 ± 42.91 | 856.91 ± 14.31 | 613.42 ± 64.17 | 24.98 ± 1.02 | 0.67 ± 0.07b | 3.91 ± 0.88b | 579.24 ± 29.98 b | 0.96 ± 0.45 b | 526.21 ± 79.13 b |
| E2 | 200.14 ± 8.31 | 837.33 ± 39.56 | 838.27 ± 51.55 | 681.03 ± 36.16 | 20.1 ± 0.91 | 0.61 ± 0.23 b | 3.89 ± 0.97b | 484.77 ± 90.41b | 0.81 ± 0.31b | 479.45 ± 68.82 b |
aBlood sugar (mg/dL), numerical density (Nv; per mm3) and total number of beta cells (×106), volume density (Vv; %) and total volume of the pancreatic islets (mm3), primary and final volume of pancreases (mm3), shrinkage ratio (%), weights of the rats (g) and their pancreases (mg), in each group: C1 as non-diabetics, C2 as not treated diabetics, E1 as diabetics with 200 mg/kg Salvia, and E2 as diabetic rats with 400 mg/kg Salvia (n = 12).
b P ≤ 0.05 vs. C2 group.
c P ≤ 0.05 vs. C1 group.
5. Discussion
The increasing prevalence of DM is a worldwide problem that may result in several irrecoverable consequences for individuals, healthcare system, and societies (26). Currently, therapeutic approaches regarding DM are mainly comprised of adjusting patients’ diet and lifestyle of patients as well as prescribing oral hypoglycemic drugs and/or insulin (27). Current therapeutic prescriptions such as sulfonylureas, meglitinides, biguanides, thiazolidinediones, and insulin may have several adverse effects, for instance, skin infection at the injection site, risk of hypoglycemia, weight gain, fluid retention, and congestive heart failure (28, 29).
Previous studies suggested that the therapeutic administration of several herbal medicines for DM could be safe (30-32). Besides, today’s trend for the use of natural plant-derived medicines encouraged researchers to conduct investigations in this regard. Among these plants, we believe SVO should also be investigated, as it consists of polyphenols that have been found to have anti-inflammatory and anti-oxidative effects.
Streptozotocin has been prescribed in several ethnomedicines for its anti-diabetic properties, which could be explained by its regulating effect on oxidative stress (33, 34). Moreover, SVO contains phenolic compounds such as Rosmaranic acid that have been shown anti-oxidative properties (35). Previous studies confirmed that this plant contains Ursolic acid that could improve inflammation and oxidative stress (36, 37). In the present study, hydro-alcoholic extract of SVO was used in STZ-induced diabetic rats that confirmed the protective effects of this agent against pancreas degeneration. Prior to our study, Eidi et al. have also shown significant reduction in triglycerides, total cholesterol, uric acid, creatinine, alanine aminotransferase, aspartate aminotransferase, and serum BS, besides an increased plasma insulin level could happen after administration of SVO in STZ-induced diabetic rats (17). Similarly, Belhadj et al. mentioned that SVO oil could alleviate metabolic problems by lowering the glycemia and increasing the glycogen storages in diabetic rats (38). Consistently, it has been shown that the administration of SVO extract exhibits the same outcomes compared to rosiglitazone; as it could increase insulin sensitivity, and decrease lipogenesis in animal models (39). Our study also showed a decrease in BS of STZ-induced diabetic rats after oral administration of the extract. Furthermore, volume density of the islets and beta cells, volume density, and the total number of beta cells were higher in SVO-treated groups. Other reports were comparably in favor of this hypothesis that SVO extract could be beneficial regarding the treatment of DM and its complications (40, 41). A recent study showed that the SVO could reduce the aldose reductase activity, which plays a crucial role in the pathogenesis of DM complications (42). Another in vitro investigation also demonstrated that SVO extract has inhibitory effects on α-glucosidase and α-amylase activities that may control the postprandial hyperglycemia (43). Furthermore, the clinical evidence was also in favor of administrating the agent for DM cases, as the consumption of SVO reduced insulin resistance in polycystic ovary syndrome patients (44).
However, in contrast to the mentioned findings, a study mentioned that intraperitoneal injection of SVO extract did not show any anti-hyperglycemic effect in sub-chronic diabetic rats (45). Another report showed controversial results as intraperitoneal injection of SVO extract significantly decreased fasting BS in normal and mild-diabetic rats, but it was not found beneficial regarding the severe condition of DM in animal models (46). Our study demonstrated that SVO-treated groups had a significant delay in the degeneration of pancreas. SVO-treated groups had higher beta cells population and the islets in comparison to non-treated group.
This study had some limitations to be considered. The study was designed to stereologically analyze the effect of SVO extract on animal models; hence, we did not test the rats regarding other inflammatory factors, which could have been helpful in the studies done on DM. Examiners’ inaccuracy may also result in bias regarding the stereological evaluation of pancreases. Moreover, we did not test the possibility of SVO toxication at higher dosages, which could be investigated in future examinations.
We believe as the progression of DM is markedly slower in our animal study, prescription of SVO as a therapeutic food supplement could be beneficial for high-risk patients. This could reduce the risk of pancreas degeneration and increase the beta cell functions. Considering the former findings regarding the effectiveness of SVO extract in DM, we hypothesize that this plant-derived medicine could have more preventive role than therapeutic. However, more animal and clinical investigations are needed to prove this hypothesis.