1. Background
The bleomycin (BLE)-containing chemotherapy regimen is widely used for drug responsive malignant cancers, such as lymphoma and germ-cell tumors. However, the adverse effects of BLE are a cause of life-threatening problems in 10% of patients. Pulmonary toxicity, known as bleomycin-induced pneumonitis (BIP), is of particular concern. Approximately 6.8% of patients with germ-cell tumors receiving BLE experience BIP (1). In developing countries such as Indonesia, people diagnosed with cancer often combine their chemotherapy treatments with alternative medicine without informing their healthcare providers. Nearly 80% of cancer patients in Indonesia use herbal as well as probiotic medicines alongside standard therapy (2).
The mechanisms by which BLE affects the lung in cases of BIP are believed to involve oxidative damage, repression of the deactivating enzyme bleomycin hydrolase, genetic susceptibility, and the accelerated production of inflammatory cytokines. Matrix metalloproteinase (MMP-1) is strongly expressed in idiopathic pulmonary fibrosis. One study showed that MMP-1 plays a role in releasing molecular precursors such as pro-transforming growth factor (TGF)-α, other epidermal growth factor (EGF)-like ligands, and TGF-α from cell surfaces or the extracellular matrix (3). An elevated MMP-1 expression has also been documented in lung disorders. However, declined level of MMP-1 is required for normal tissue remodeling, while elevated levels of MMP-1 have been found in chronic non-healing wounds (4).
Kefir is a milk-fermented product with many health benefits. It is commonly made from cow milk, but it can also be produced from goat milk containing a mixture of lactic acid, fat, amino acid, lactose, protein, Vit B complex, Vit A, acetaldehyde, acetoin, low concentration of alcohol, and other fermentation flavor products (5, 6). Several studies have demonstrated that kefir has immunomodulatory effects by increasing the production of TNF-α and other cytokines, such as IL-5, IL-6, and IL-12 (7). Recent studies have suggested that kefir has the potential to kill cancer cells in vitro (7, 8). Hence, cancer patients may benefit from consuming kefir as a complementary traditional medicine alongside standard chemotherapy. However, more investigation is needed, particularly about its potential side effects (9). Currently, the number of studies showing the negative effects of kefir in patients receiving BLE as part of their chemotherapy regimen is limited.
2. Objectives
This study aimed to investigate the effect of administering high doses kefir in BLE-induced rats. We were particularly interested in the effect kefir might have on the development of lung fibrosis.
3. Methods
3.1. BLE Rat Model
All protocols concerning the use of rats were approved by the Commission on Health Research Ethics of the Faculty of Medicine, Brawijaya University, Malang, Indonesia (39/EC/KEPK /01/2017). Wistar male rats were purchased from the Department of Biology at the Islamic University in Malang and allowed to acclimatize in cages (n = 6 per cage) for 1 week. Inclusion criteria were healthy and active Wistar male rats (aged 16 weeks; weight between 150 - 200 g), which received no chemical treatment prior to the study. Rats that were not eager to eat, suffering from disease, or handicapped during the course of the experiment were excluded from the study. A total of 30 rats were divided into five equal groups (n = 6 each). Group I was treated with phosphate buffered saline (PBS) and served as negative control; group II was given BLE; groups III, IV, and V were given BLE and 2.5 mL, 3.5 mL, and 4.5 mL of kefir, respectively. Rats received kefir or PBS via oral gavages on a daily basis for 30 days, which was counted from the first day of BLE administration. A 0.9% solution of BLE (Nippon Kayaku, Kalbe Farma, Jakarta, Indonesia) based on 2 mg/kg body weight in 100 μL of saline was prepared and stored in aliquots at 4°C until needed (10). Bleomycin hydrochloride was administered intranasally at a dose of 50 μL per nostril on experimental days 1, 3, 5, 7, 9, 10, and 13. During this process, the rats were lightly anesthetized using 0.05 mL of 10% ketamine (Kepro B.V., Deventer, Netherlands) and xylazine (0.5 mg/200 mg BW of rat) (Interchemie, Venray, Netherlands) by intramuscular injection. This procedure was performed once daily over 9 consecutive days. An administration protocol has been used previously to generate successful sustained pulmonary fibrosis in mice (5, 6). On day 30, all rats were sacrificed by cervical dislocation. Blood samples from each rat were drawn into vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). Plasma was collected by centrifugation (15 min at 3000 rpm) and aliquoted into 0.5 mL, which were then stored at -20°C for enzyme-linked immunosorbent assays (ELISA). Lung tissues were collected for histological analysis.
3.2. Kefir Sample
Plain kefir was purchased from a small firm in Malang, Indonesia, aliquoted upon receipt, and rapidly frozen at -80°C until needed.
3.3. Histology
The inflation of lungs was conducted with injection of 4% paraformaldehyde in PBS intratracheally at 25 cm H2O static pressure for 1 h. They were immersed in the same buffer overnight at 4°C. On the next day, the lungs were processed after trimming for histology. Hematoxylin and eosin (H&E) staining was used to stain the tissue sections.
3.4. Immunohistochemical Assays
Lung tissue was immersed in 10% neutral buffered formalin for 24 h. Following fixation, lungs were trimmed to three transverse sections with 0.3 cm thickness. Sections were dehydrated using ethyl alcohol and then embedded in a block of paraffin wax. Three histological slides (4 µm) were processed for immunohistochemistry (IHC) using α-smooth muscle actin polyclonal antibodies (Wuhan Fine Biotech Co. Ltd., Hubei, China). Alpha smooth muscle actin-stained slides were examined with a light microscope (Eclipse E200 LED, Nikon Ex, Tokyo, Japan) connected to a color photomicrography digital camera system (DS-Fi2-L3, Nikon Ex, Tokyo, Japan). A x10 objective lens was used to randomly select fields. Program for image analysis software was employed to measure the area of all stained tissue and divide it by the constant field interest area.
3.5. Measurement of Cytokines
Plasma was assayed using solid-phase ELISAs. The rat IL-6 ELISA kit (Abcom, Cambridge, MA, USA) and the TNF-α Quantikine ELISA Kit (R&D systems Inc. Minneapolis, MN, USA) were used. The ELISA test was performed according to the manufacturer’s guidelines. Results were read by an iMark™ Microplate Absorbance Reader (Bio-Rad, Hercules, CA, USA).
3.6. Immunostaining Assay for MMP-1and STAT-3
Histochemical analysis of deparaffinized MMP-1 and STAT-3 expression was conducted using antibodies and antisera against MMP-1 and STAT-3. Rat-lung tissue sections were collected on silanate glass slides and then deparaffinized and rehydrated. Following the inhibition of endogenous peroxidase activity, tissue sections were incubated 12 hours at 4°C with primary antibodies diluted to 1: 500 using Dako antibody diluent (Agilent, Carpinteria, CA). The cytomation LSAB+ system CRF anti-polyvalent HRP polymer (Agilent, Carpinteria, CA) was used to detect the reactions using diaminobenzidine tetrahydrochloride as the chromogen substrate (Dako Cytomation, Liquid DAB, Agilent, Carpinteria, CA). The primary antibody was detected using biotinylated rabbit antirat Ig (Vector Laboratories Inc., Burlingame, CA, USA) at a 1:500 dilution. Visualization of bound antibodies was done using ABC peroxidase (Vector Laboratories Inc., Burlingame, CA, USA). Following counterstaining of the sections with methyl green, production of color photomicrographs was done with a Nikon E600 photomicroscope and processed as described earlier. Adjacent serial sections stained in the absence of the primary antibody served as negative control.
3.7. Statistical Analysis
All the data measurements were expressed as mean ± SEM. Statistical analyses were performed using SPSS 21.0 software. The TNF-α and IL-6 endpoints were analyzed using parametric one-way ANOVA and least significant difference (LSD) test. Non-parametric Kruskal-Wallis test and Mann-Whitney U test were performed for the STAT-3 and MMP-1. The differences were considered significant at P < 0.05. The following abbreviations were used for indication of significance: *: 0.01 < P < 0.5; **: 0.001 ≤ P ≤ 0.1; ***: P < 0.001.
4. Results
4.1. Histology
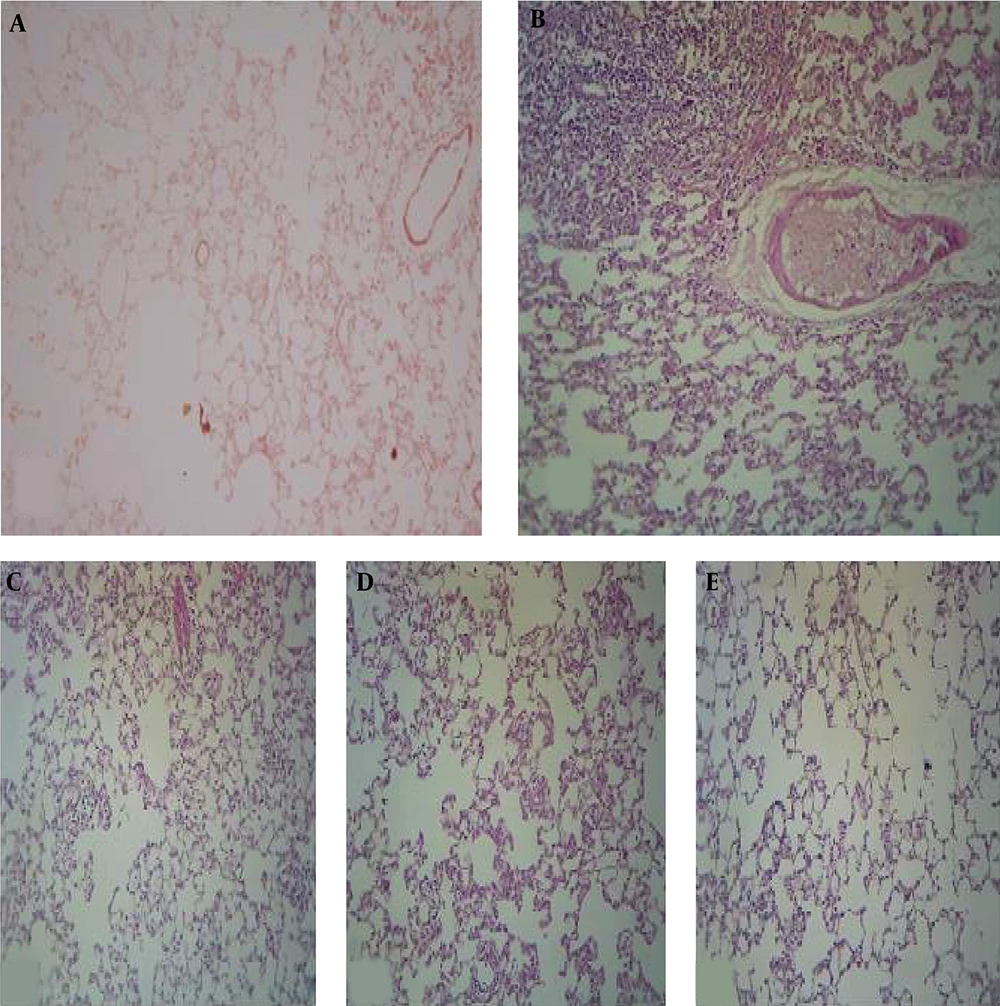
Histological examinations showed significant differences between the control and BLE-only group (Figures 1A and B). The controls exhibited normal lung tissue. In contrast, the lungs of the BLE group exhibited typical findings associated with idiopathic pneumonitis (IP). Most of the alveoli were filled with lymphocytes, although some neutrophils were observed, indicating both acute and chronic inflammations. Thickenings were also identified in various areas, with fibroblasts in the extracellular matrix, indicating fibrogenesis. Within the vessels, we found extravasation of inflammatory cells in the lung tissue, indicating edema.
Compared to the controls, the BLE groups supplemented with kefir exhibited changes ranging from mild to moderate infiltration of cells in the alveolar tissue, with some fibrosis detected according to the dose of kefir received (Figures 1C - E). Generally, modest inflammatory changes were found and observed as infiltrations in limited areas of the alveolar lumina and septa. Lymphocytes filled the interstitial tissue space such that the alveolar walls were destroyed in some places and thickened in others due to the infiltration. Likewise, there were slight depositions of collagen in the extracellular matrix indicating pulmonary fibrosis. In general, following BLE and kefir exposure, the lung cells exhibited high rates of apoptosis and infiltration, suggesting that the administration of kefir may have increased these processes.
4.2. MMP-1 Cellular Expression
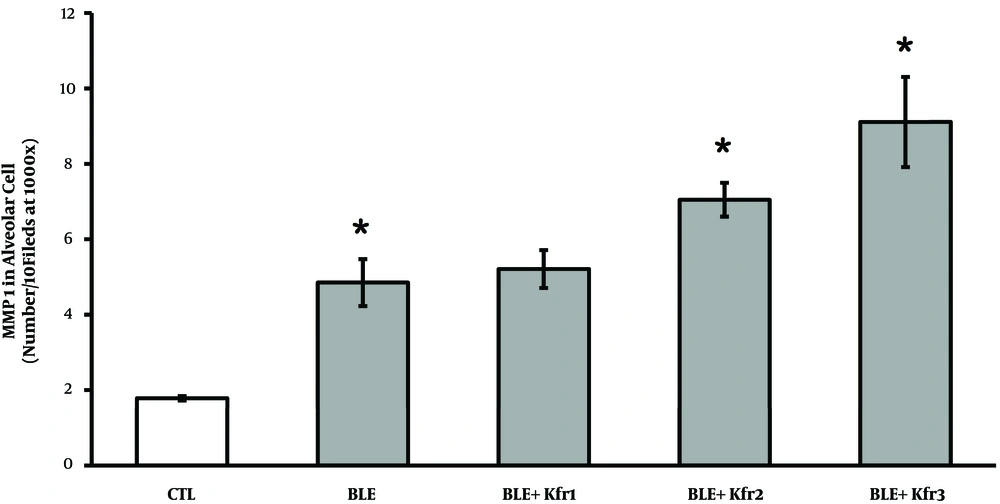
IHC results demonstrated a significantly elevated expression of MMP-1 in all the BLE groups (Figure 2). Rats receiving 2.5 mL kefir showed a higher number of cells expressing MMP-1, which was significantly different from the control group (P = 0.023, P < 0.05). As the dose of kefir increased from 3.5 to 4.5 mL, the synthesis of MMP-1 also significantly increased (P = 0.025).
Immunohistochemistry of MMP-1 expression by alveolar cells of bleomycin-exposed rats administered by different doses of kefir. CTL (control); 2.5 mL (Ble + kfr1); 3.5 mL (Ble + kfr2); and 4.5 mL (Ble + kfr3). All doses of kefir (2.5, 3.5, and 4.5 mL) significantly increased (P = 0.025) the number of cells expressing MMP-1. * Indicates that there is a significant different between treatment and negative control (P < 0.01).
4.3. TNF-α Plasma Levels
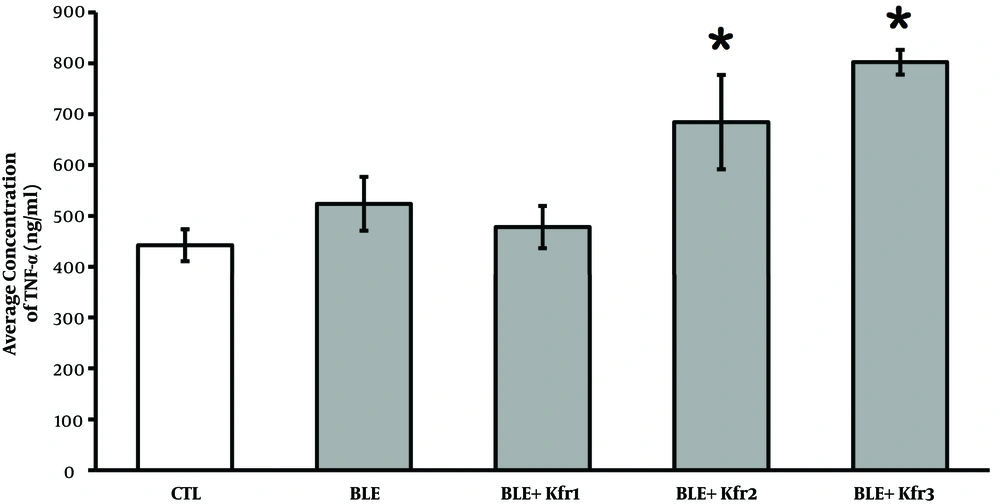
Circulating TNF-α levels were slightly higher in the BLE-only group compared to the controls. Following the administration of 2.5 mL of kefir, the TNF-α concentration rose in comparison to the controls, though it was not statistically significant (P = 0.177, P > 0.05). However, the administration of 3.5 and 4.5 mL of kefir significantly (P = 0.034, P < 0.05) increased the level of TNF-α (Figure 3).
Plasma TNF-α levels at different kefir doses in bleomycin-exposed rats. CTL (control); 2.5 mL (Ble + kfr1); 3.5 mL (Ble + kfr2); and 4.5 mL (Ble + Kfr3). High dose kefir (3.5 and 4.5 mL) significantly (P = 0.034) increased the number of cells expressing TNF-α. * Indicates that there is a significant different between treatment and negative control (P < 0.01).
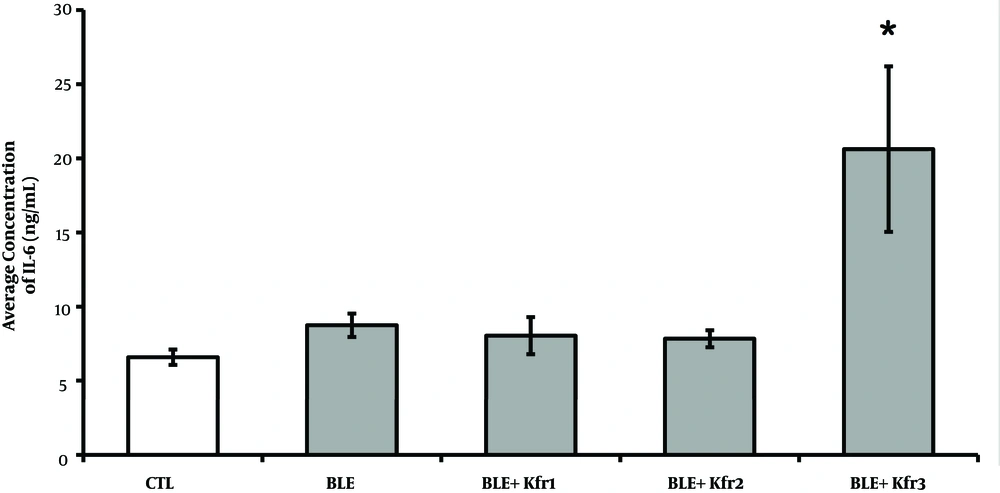
4.4. IL-6 Plasma Levels
The BLE group exhibited a higher concentration of IL-6 compared to the control group. The groups given 2.5 and 3.5 mL of kefir were not significantly different from the BLE group. However, only the 4.5 mL kefir group exhibited significant elevated IL-6 plasma levels when compared with all other groups (P = 0.004, P < 0.05) (Figure 4).
IL-6 plasma concentrations at different kefir doses in bleomycin-exposed rats. CTL (control); 2.5mL (Ble + kfr1); 3.5 mL (Ble + kfr2); only highest dose kefir 4.5 mL (Ble + kfr3) group exhibited drastically elevated IL-6 plasma levels (P = 0.004). * Indicates that there is a significant different between treatment and negative control groups (P < 0.01).
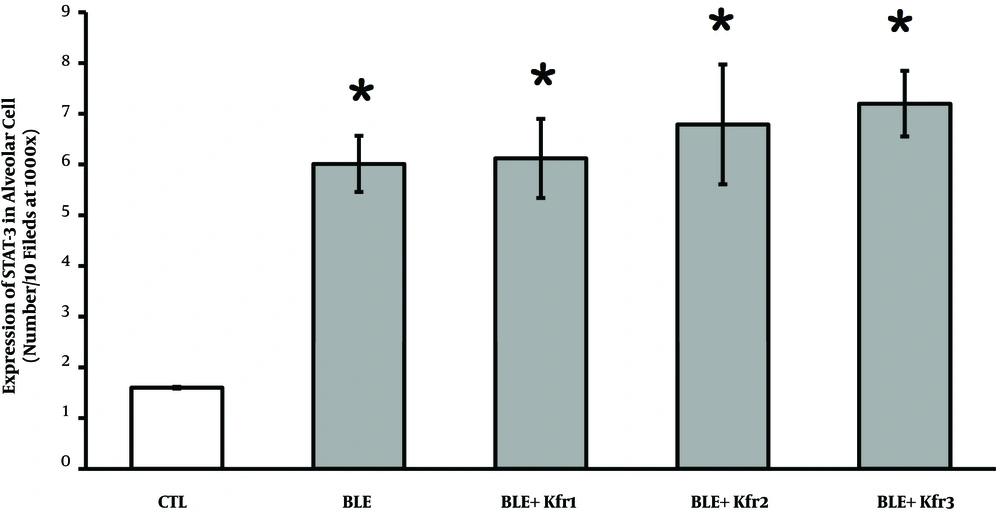
4.5. STAT-3 Expression
Using IHC method, the expression of STAT-3 was qualitatively evaluated. The number of alveolar cells expressing STAT-3 indicated that BLE exposure significantly raised this number. This increase was seen across the groups administered with kefir, which demonstrated a significant increase (P = 0.034, P < 0.05) compared to the control group (Figure 5).
Immunohistochemistry of the expression of STAT-3 in alveolar cells in bleomycin-exposed rats and kefir-treated rats. 2.5 mL (BLE + Kfr1); 3.5 mL (BLE + Kfr2); and 4.5 mL (BLE + Kfr3). All groups treated with kefir showed significant increase compared to negative control (P = 0.034). * Indicates that there is a significant different between treatment and negative control groups (P < 0.01).
5. Discussion
This study aimed to investigate the effects of kefir consumption on pneumonitis associated with BLE. Rats exposed to BLE showed lung damage marked by histopathological changes dominated by the formation of infiltrate in alveolar cells and some extracellular matrix thickening. In BIP, lung tissue undergoes a series of events facilitated by the communication between cells and the extracellular matrix during the recovery process. Failure of this process would then result in the excessive deposition of matrix components. However, despite the formation of pulmonary fibrosis, lung tissue enlargement could also occur as an end result.
In response to inflammation, a number of different cell types express MMPs, of which MMP-1 plays an important role in tissue remodeling, wound healing, and inflammation (11). Despite the requirement of MMPs for the recruitment of leukocytes to sites of injury and infection, a high level of MMP activity has been involved in matrix breakdown and tissue remodeling in lung diseases (12). This could account for the strong expression of MMP-1 in the pulmonary epithelial cells observed in this study.
Previous studies have shown that the administration of BLE to induce lung injury correlates with increased expressions of TNF-α and IL-6 as part of a cytokine network that stimulates pro-fibrotic inflammatory lesions (13, 14). TNF-α could also lead to the development of alveolar enlargement and loss of elastic recoil (15). In our study, serum levels of TNF-α in BLE-exposed lung tissue in the groups with the two highest doses of kefir (3.5 and 4.5 mL) were much higher than in the BLE-only group. These elevated TNF-α levels could be due to sphingomyelin, an active compound of kefir, that may play a role in the induction of TNF-α (16). Kollias (17) found excessive TNF-α levels in idiopathic pulmonary fibrosis cases. This is similar to our histological findings that demonstrated both slight fibrosis and some lung tissue enlargement. Transgenic mice that overexpress TNF-α do not develop pulmonary fibrosis, but they do develop alveolar enlargement and loss of elastic recoil, which are characteristics of pulmonary emphysema (18). Hence, TNF-α by itself may not be able to induce pulmonary fibrosis, but it may accentuate the fibrotic process in conjunction with BLE or silica.
In group administered with kefir, both TNF-α and IL-6 are up-regulated. Along with inflammation, lung injury following BLE administration stimulates both of these cytokines. IL-6 activates a variety of pro-inflammatory and pro-fibrotic responses in BLE-exposed rat models, depending on which organ is exposed (19). Although not proven in vivo, the consumption of kefir in large doses could increase IL-6, which would accelerate the change from sub-chronic lung conditions to fibrosis.
IL-6 can also induce STAT-3 activation (20). Phosphorylated by various kinases, STAT-3 is implicated in aberrant fibroblast activation in fibrotic diseases. Thus, STAT-3 might be an important molecular checkpoint for tissue fibrosis (21). In idiopathic pulmonary fibrosis (IPF) patients and BLE-exposure animal models, intratracheal surges in the levels of phosphorylated STAT-3 have been found (22). In our study, the results from the kefir plus BLE groups suggested that high doses of kefir stimulated STAT-3 expression and increased IL-6 concentration. However, we cannot be certain from the histological examinations that fibrosis occurred, as the Aschraf scale gave a value of 4.5 (data not shown), suggesting the tissue was in an initial and sub-chronic condition. This is the limitation of this study.
This is the first study to show that kefir can induce both MMP-1 and TNF-α and that these inductions may be correlated. Bacteria such as Lactobacillus sp. might play a large role in this induction. While previous studies demonstrated that the bacteria in kefir can accelerate wound healing (23), our study results do not support this finding. This may be explained by the differences in the amounts of kefir used. Therefore, diets that modulate immune system responses in the intestine may produce a strong inflammatory response, especially in individuals undergoing cancer therapy (24).
In developing countries, many cancer patients consume probiotics, including kefir, as a complement to standard drug treatments. For probiotics, the risk of an inflammatory process should be considered if they are consumed in high doses. This is the first study in Indonesia conducted on BLE-exposed rat model, suggesting that consumption of kefir in high doses has potentially harmful effects. Therefore, we recommend that cancer patients treated with BLE avoid consuming probiotic supplements as they may increase the risk of BIP.
5.1. Conclusion
The results of our study on BLE-exposed rats demonstrated that consumption of kefir in high doses has a less beneficial effect. High doses of kefir modulate and increase the expression of plasma IL-6 and STAT-3 on alveolar cells, that may accelerate the change from sub-chronic lung conditions to fibrosis.