1. Background
Nowadays, demand for medicinal plants is considerably increasing in developing and developed countries. The use of herbal medicines, known as folk remedies, is common worldwide, with a growing trend, to treat known diseases and problems, such as diabetes, common cold, obesity, anxiety, eczema, parasitic, and microbial infections (1-3).
Teucrium L. (Teucrium polium L., Teucrium capitatum L., germander) resides in the Lamiaceae family and has a long history of consumption among medicinal plants. Teucrium L. is a traditional remedy and native to South-Western Asia, North Africa, Europe, and Iran. This genus with 12 species is identified in various regions of Iran. Teucrium polium L (T. polium) is one of the most common species with global spread (4-6).
Teucrium polium is a perennial shrub with linear and oblong leaves and small flowers ranging from white to pink. It contains terpenoids, glycosides, iridoids, saponins, polyphenols, flavonoids, and furano neoclerodane diterpenoids. Its brew is usually consumed to treat common cold, abdominal colic, indigestion, type 2 diabetes, and wounds. Because of the various usage of T. polium in folk medicine, it is one of the most widely used plants (7-10).
Despite the wide medicinal use of T. polium, some studies claimed its toxicity, especially after chronic consumption; for example, hepatotoxicity, neurotoxicity, and even carcinogenicity (11-15). A recent study on the effect of T. polium revealed significant changes in the kidney tissue of mice treated with this herb; disturbance in the kidney function was also reported (14). Another study by Laliberte and Villeneuve (11), demonstrated that chronic consumption of T. polium led to hepatitis. Moreover, Al-Ashban et al. (15), reported that long-term use of T. polium in mice could induce damage to sperm structure and function.
Toxins may also affect some cellular markers, such as Ki-67, the glial fibrillary acidic protein (GFAP), p53, and CD31. Ki-67, a nuclear antigen, is a marker of cellular proliferation whose expression accounts for cell growth and survival (16). The GFAP is a reliable indicator for astrocytes activity, which has a diagnostic value in neuropathology changes (17). In addition, p53 is an effective marker to determine toxicity, apoptosis, cellular stress, or growth arrest (18). CD31, known as platelet endothelial cell adhesion molecule 1 (PECAM-1), is a marker for vascular differentiation (19).
2. Objectives
In the present study, the researchers investigated the probable effects of T. polium crude extract and various fractions on the brain, liver, and kidney tissue in the subchronic phase using an animal model.
3. Methods
3.1. Extract Preparation
The study protocol was approved by the Ethics Committee at Kerman University of Medical Sciences, Kerman, Iran (ethical code: 93/23). Teucrium polium was collected from Khabr County in Kerman Province and was confirmed at the Department of Pharmacognosy, School of Pharmacy, Kerman University of Medical Sciences. The specimen was deposited in the herbarium with voucher specimen no.: 28125. Dried aerial parts of T. polium were powdered and soaked in 80% ethanol for 24 hours. The ethanol extract, after filtration, was evaporated using a rotary evaporator at 50°C. The crude extract (TPCE) was reconstituted with diethyl ether (DE) and petroleum ether (PE), and then the fractions were re-evaporated under vacuum to dry. The crude extract, DE, and PE fractions were dissolved in dimethyl sulphoxide (DMSO) to be used during experiments.
3.2. Animals and Groupings
In the present experimental study, 45 adult male Wistar rats, with an average weight of 200-250 g and age of 8 - 10 weeks, were used. The animals were housed under standard conditions (room temperature 23°C - 25°C, humidity 45% - 55%, and 12:12-hour light-dark cycle) with free access to water and food. Rats were randomly assigned to five experimental groups (n = 9) as PBS (receiving 100 µL phosphate-buffered saline), vehicle (receiving 100 µL (10 µL DMSO + 90 µL PBS as a solvent of the extract and fractions), as well as CE, PE, and DE receiving 3 mg/kg/day (100 µL) TPCE, PE, and DE, respectively for six weeks through intraperitoneal injections.
3.3. Hematoxylin-Eosin and Immunohistochemistry Staining
The animals were deeply anesthetized with a mixture of ketamine/xylazine and perfused with PBS (PH = 7.4) followed by 10% formaldehyde. Their brain, liver, and kidney were removed and fixed in 10% formalin to examine the histological changes. After paraffin impregnation, 3-µm‐thick sections were provided. The slides of liver and kidney tissue, as well as brain sections, were stained with hematoxylin-eosin (H & E). The researchers focused on pathological changes, including vacuolization of neurons, degenerated neurons, foamy cells, apoptotic cells, and vacuolar spaces in the brain. Also, degeneration, apoptosis, vacuolization of cells, and other changes were assessed in the liver and kidney tissue. At least 20 microscopic fields were assessed for any pathological changes per sample at 400× magnification by a light microscope (Olympus IX51, Japan).
3.3.1. Immunohistochemistry Study of the Brain Tissue
Some sections of the brain were stained immunohistochemically by the streptavidin-biotin method. The brain was stained using monoclonal mouse anti-human P53 antibody (Santa Cruz Biotechnology, USA. 1:50), monoclonal mouse anti-human Ki-67 (Dako, Carpinteria, CA, USA. 1:100), monoclonal mouse anti-human Nestin (Santa Cruz Biotechnology 1:200), monoclonal mouse antibody against GFAP (Santa Cruz Biotechnology, USA 1:200) and PECAM-1 monoclonal mouse anti-rat CD-31 (Bio-Rad 1:50). The positive reaction against each antibody was assessed in 10 microscopic fields at 400× magnification (20).
3.4. Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). Firstly, the normality of data was examined using the Kolmogorov-Smirnov test. Data were analyzed by one-way ANOVA using the Tukey post hoc test for multiple comparisons (SPSS version 16). The difference among the groups was considered significant, with a P-value of < 0.05.
4. Results
4.1. Histological Changes of the Brain
4.1.1. The Study of H&E-Stained Sections
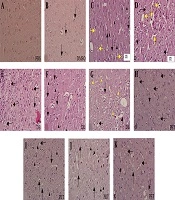
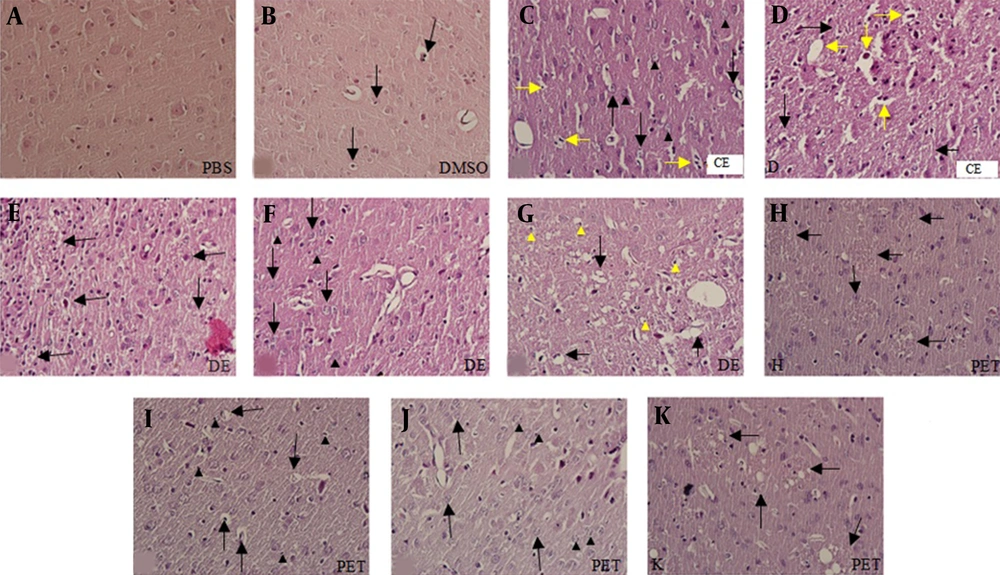
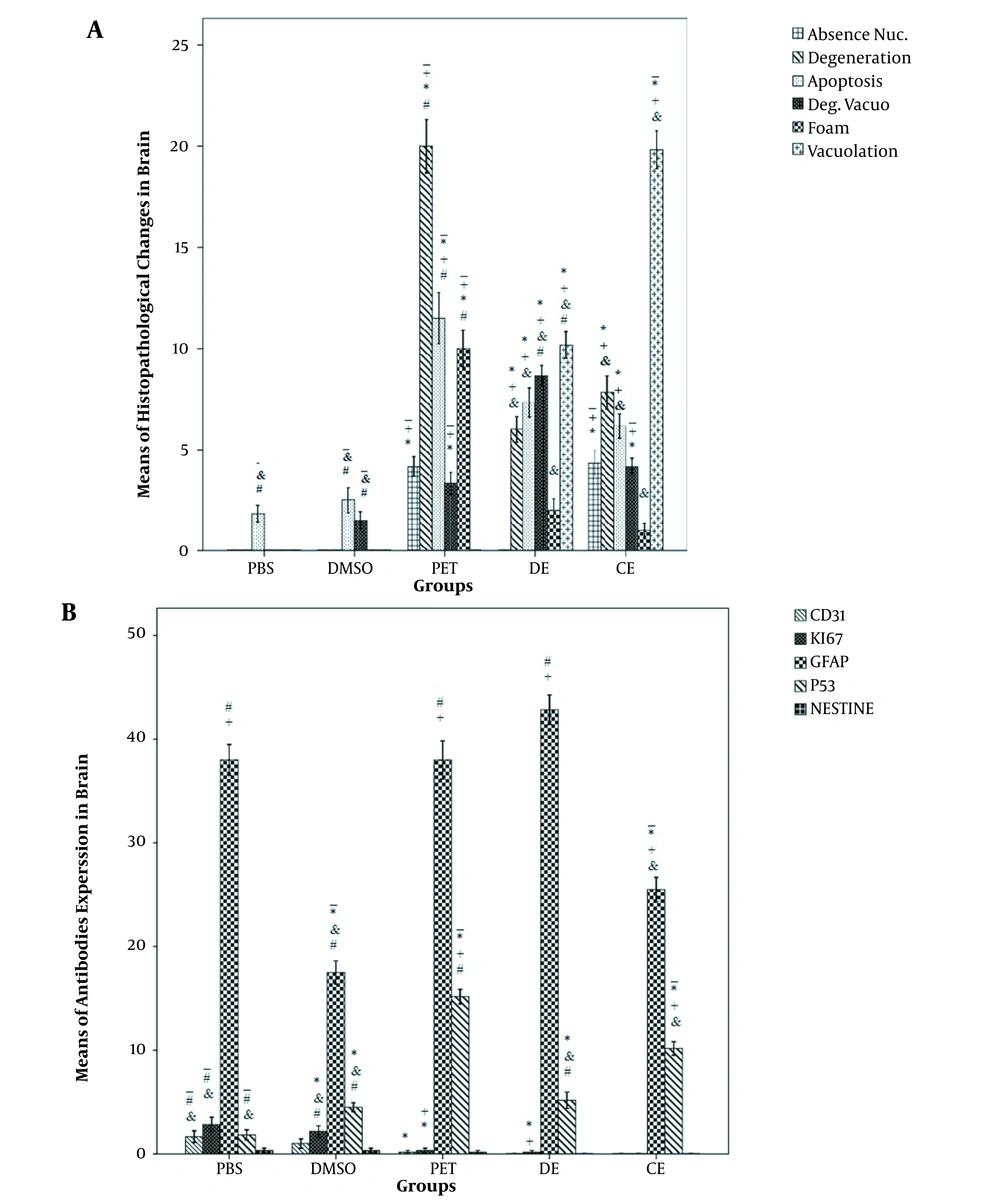
Histological study of H & E-stained slides of the brain revealed the following changes: in the PBS group, brain tissue was characterized by normal histoarchitecture. All of the neurons and glial cells had normal shape and size. However, a few apoptotic cells were seen in this group (Figure 1A). In the DMSO group, brain structure was normal, but apoptosis was observed in a few cells. Moreover, a few degenerating neurons with vacuolization were seen (Figure 1B). In the CE group, some degenerating neurons were observed. The vacuolar spaces were detected around the normal cells, and an increase in apoptotic cells was found in comparison with the PBS and DMSO groups. In the CE group, some degenerated vacuolar and foamy cells were observed (Figure 1C and D). In the DE group, the vacuolar spaces around the glial cells and neurons were significantly less than those in the CE group (P < 0.003). However, the number of degenerated vacuolar cells was significantly higher than those of other groups (P < 0.001). In addition, apoptotic, degenerated, and foamy cells were observed in this group; however, there were no significant differences with those of the CE group (Figure 1E-G). In the PE group, the number of degenerated cells with pale nuclei was remarkably higher than those of other groups (P < 0.01). Besides, apoptosis in the glial cells and neurons (intensely colored nuclei with hematoxylin) was considerably abundant in this group (P < 0.001). Foamy cells with large vacuoles were clearly seen in the PE group. The number of foamy cells in this group was significantly higher than those of other groups. However, a significant reduction was observed in the number of degenerated vacuolar cells in the PE group in comparison with the DE group (Figure 1 H-K and 2A).
Photomicrographs of rat brain sections stained with H & E (A-K) at 400× magnification. Figure A shows the PBS group and figure B, apoptosis (black arrows) in the DMSO group; C and D histopathological changes in the CE group; C, degeneration (black arrowheads), apoptosis (yellow arrows), and vacuolar spaces (black arrows); D, foamy cells (yellow arrows) and degenerate vacuolar cells (black arrows). Figures E-G show histopathological changes in the DE group; E, apoptosis of neuron (black arrows); F, apoptosis (black arrows) and degeneration (black arrowheads); G, foamy cells (black arrows) and vacuolar spaces (yellow arrowheads). Figures H-K show histopathological changes in the PE group; H and K, foamy cells (black arrows); I, vacuolar spaces (black arrows) and apoptosis (black arrowheads); J, cells without a nucleus (black arrowheads) and degenerated cells (black arrows).
4.1.2. Immunohistochemistry Study of the Brain Tissue
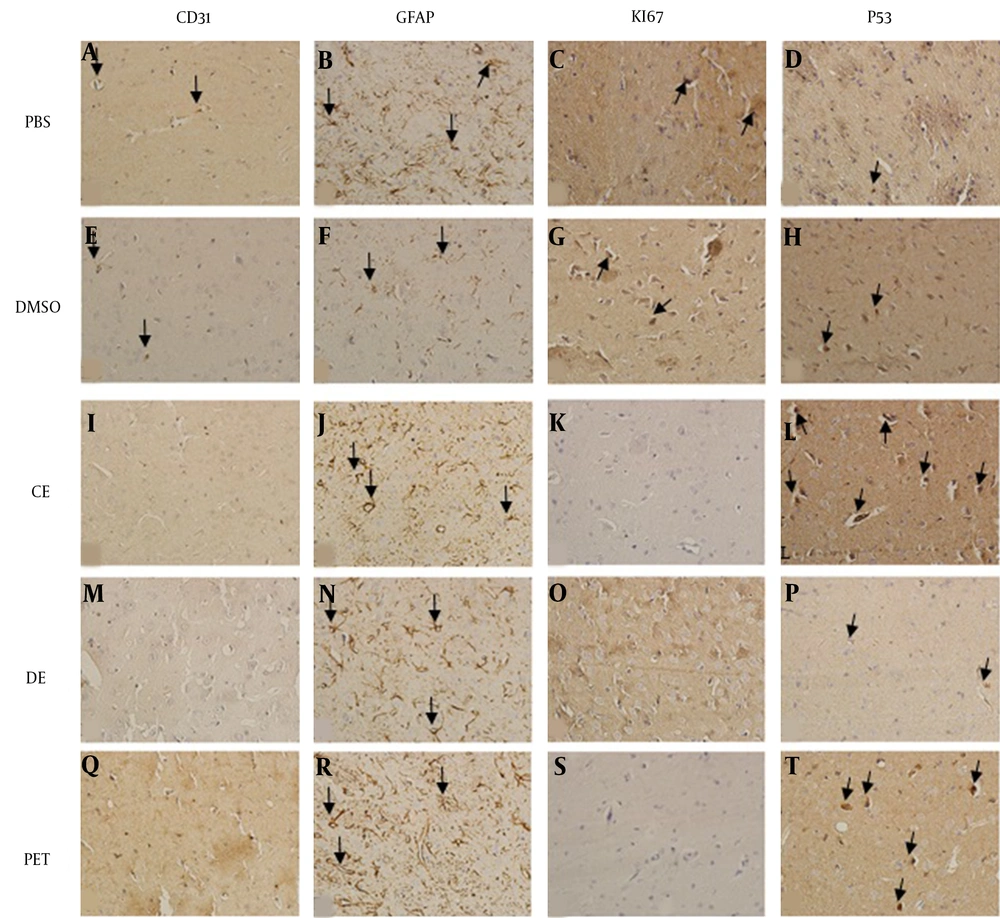
In the PBS, DMSO, and PE group slides, some positive reactions against CD31 and Ki67 were detected, while it was absent in other groups. Moreover, the number of CD31- and Ki67-positive cells in the PBS group was significantly higher than those of the PE, CE, and DE groups. Both highest and lowest numbers of GFAP-positive cells were observed in the PE and DMSO groups, respectively. The CE group had a weaker positive reaction compared with PBS and DE groups. The number of GFAP-positive cells was significantly lower in the CE and DMSO groups compared with those in other groups (P < 0.001). A statistically significant number of P53-positive cells was also detected in the PE and CE groups compared with other groups (P < 0.002). The nestin-positive cells were not observed in the DE and PE groups, while a few Nestin-positive cells were seen in the PBS, DMSO, and PE groups (Figures 2B and 3).
A, Histopathological findings of the brain tissue in the study groups; B, immunohistochemical findings of the brain in the study groups. *, A significant difference of the PBS group with other groups (P < 0.05); +, a significant difference of the DMSO group with other groups (P < 0.05); -, a significant difference of the DE group with other groups (P < 0.05); &, a significant difference of the PE group with other groups (P < 0.05); #, a significant difference of the CE group with other groups (P < 0.05)
IHC photomicrographs of rat brain sections (A-T) at 400× magnification. Figures A-D show the expression of antibodies in the PBS group; A, CD31-positive cells (black arrows); B, GFAP-positive cells (black arrows); C, KI67-positive cells (black arrows); D, P53-positive cells (black arrows). Figures E-H show the expression of antibodies in the DMSO group; E, CD31-positive cells (black arrows); F, GFAP-positive cells (black arrows); G, KI67-positive cells (black arrows); H, P53-positive cells (black arrows). Figures I-L show the expression of antibodies in the CE group; I, without CD31-positive cells; J, GFAP-positive cells (black arrows); K, without KI67-positive cells; L, P53-positive cells (black arrows). Figures M-P show the expression of antibodies in the DE group; M, without CD31-positive cells; N, GFAP-positive cells (black arrows); O, without KI67-positive cells; P, P53-positive cells (black arrows). Q-T Q-T show the expression of antibodies in the PE group; Q, without CD31-positive cells; R: GFAP-positive cells (black arrows); S, without KI67-positive cells; T, P53-positive cells (black arrows).
4.2. Histological Changes of Liver
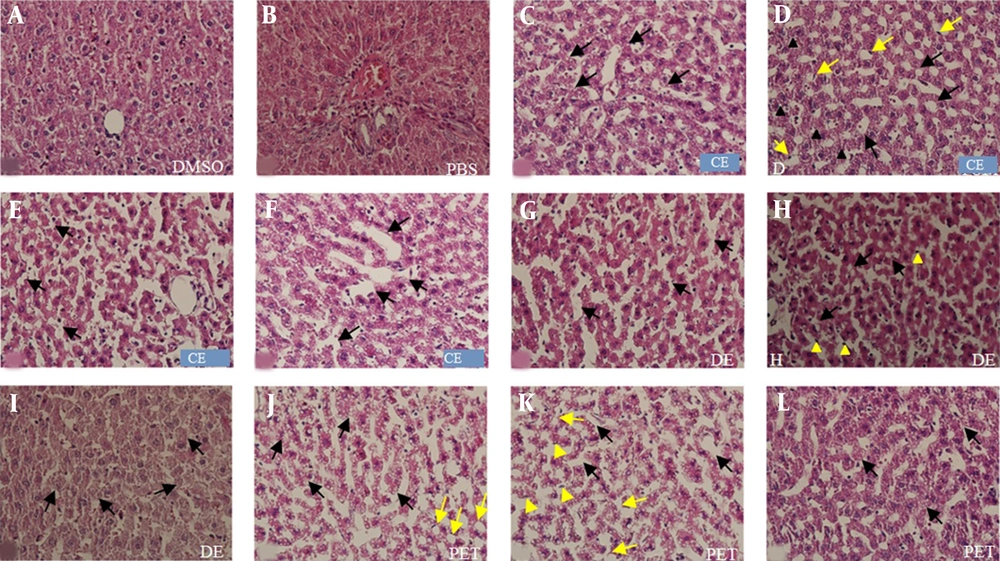
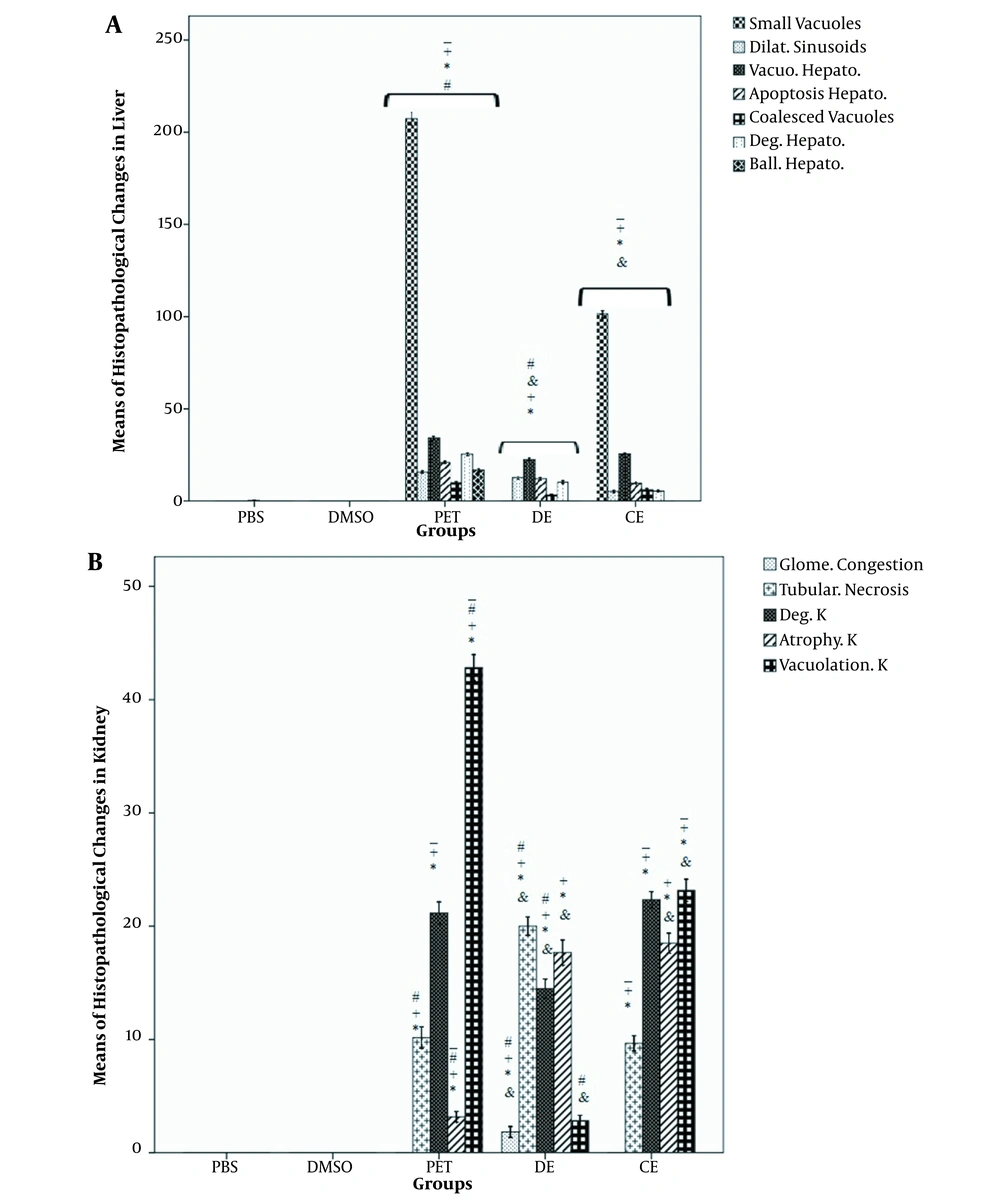
Liver slides obtained from the DMSO and PBS groups had normal hepatocytes and other liver residing cells. In the extract-treated groups, degeneration, apoptosis, vacuolization of hepatocytes, and dilated sinusoids were found. The greatest changes were detected in the PE group, followed by the DE and CE groups. Also, coalesced vacuoles were seen in the treated groups, and the liver of PE rats was found the most influenced one among the studied groups. More small vacuoles were observed in the PE and CE groups; the PE group had a significant difference with other groups; the case was not detected in the DE group. Ballooned hepatocytes were just observed in the PE group (Figures 4 and 5A).
Photomicrographs of rat liver sections stained with H & E (A-K) at 400× magnification. A shows the DMSO group and figure B the PBS group. Figures C-F show histopathological changes in the CE group; C, small vacuoles (black arrows); D, degeneration (yellow arrows), apoptosis (black arrows), and vacuolization of hepatocytes (black arrows); E, coalesced vacuoles (black arrows); F, dilated sinusoid (black arrows). Figures G-I show histopathological changes in the DE group; G, dilated sinusoid (black arrows); H, coalesced vacuoles (black arrows) and apoptosis (yellow arrowheads); I, degeneration (black arrows). Figures J and L show histopathological changes in the PE group; J, small vacuoles (black arrows) and vacuolization of hepatocytes (yellow arrows); K, dilated sinusoid (black arrows), apoptosis (yellow arrowheads), and ballooned hepatocytes (yellow arrows); L, degeneration (black arrows).
A, Histopathological findings of the liver tissue in the study groups; B, histopathological findings of the kidney tissue in the study groups. Data are expressed as mean ± SEM. *, a significant difference of the PBS group with other groups (P < 0.05); +, a significant difference of the DMSO group with other groups (P < 0.05); -, a significant difference of the DE group with other groups (P < 0.05); &, a significant difference of the PE group with other groups (P < 0.05); #, a significant difference of the CE group with other groups (P < 0.05)
4.3. Histological Changes of the Kidney
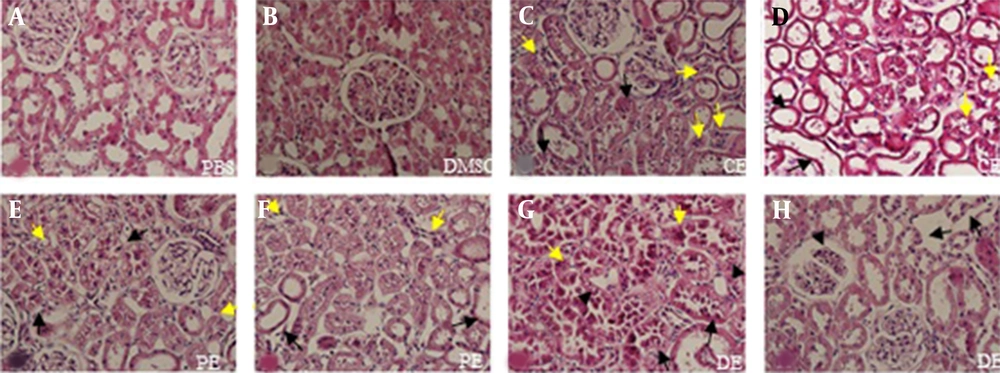
In the PBS and the DMSO groups, normal architecture of the kidney tissue was observed, whereas atrophy, degeneration, and vacuolization of renal cells were detected in the extract-treated groups. Necrosis was also observed in the tubular epithelial cells in these groups. The rate of vacuolization in renal cells was significantly high in the PE group; however, it was significantly lower in the DE group in comparison with the CE group (P < 0.01). The number of degenerated cells was significantly higher in the PE and CE groups than other groups (P < 0.002). The rate of atrophic cells in the PE group was remarkably lower than those of the CE and DE groups (P < 0.001). Besides, necrosis of the tubular epithelial cells considerably increased in the DE group in comparison with other groups (P < 0.001). The glomerular congestion was only seen in the DE group (5B and Figures 6).
Photomicrographs of rat kidney sections stained with H & E (AH) at 400× magnification. Figure A shows the PBS group and figure B DMSO group. Figures C and D show histopathological changes in the CE group; C, degeneration (black arrows) and necrosis (yellow arrows); D, atrophy (black arrows) and vacuolization (yellow arrows). Figures E and F show histopathological changes in the PE group; E, degeneration (black arrows) and vacuolization (yellow arrows); F, atrophy (black arrows) and necrosis (yellow arrows). Figures G and H show histopathological changes in the DE group; G, degeneration (black arrows), necrosis (yellow arrows), and vacuolization (black arrowheads); H, necrosis of tubular epithelium cells (black arrows) and glomerular congestion (black arrowheads).
5. Discussion
To investigate the effects of T. polium, as a medicinal herb, the present study aimed at examining the effects of long-term administration of the sub-lethal dose of crude extract, DE, and PE fractions of T. polium on the histological pattern of the brain, kidney, and liver in rats. The extracts of T. polium demonstrated adverse effects on the brain tissue. Similar results were also found in the kidney and liver tissue.
Treatment of animals with crude extract, DE, and PE fractions of T. polium resulted in apoptosis, degeneration, vacuolization of neurons, and formation of foamy cells in the brain tissue. The H&E-stained slides also suggested morphological changes in the brain tissue. Examination of nerves and glial cells also indicated that cytoplasmic vacuoles were filled with amorphous material, surrounded by a single membrane. Moreover, it was found that the extract and fraction of T. polium significantly inhibit cell proliferation and increase apoptosis and degeneration in the neurons and glial cells. Rajabalian (21) examined the cytotoxicity of the methanol extract of T. polium on seven cancerous cell lines in a combination therapy scenario. They could successfully report a higher cell death rate in the combination therapy using anticancer drugs, including vincristine, doxorubicin, and vinblastine. Sheikhbahaei et al. (22), demonstrated that a combination of PE fraction of T. polium with tranilast, an anti-angiogenesis substance, decreased angiogenesis and its related genes significantly. Cell arrest at the S phase was postulated as the main molecular mechanism for the cytotoxic potential of T. polium. Also, treatment of cancer cells with T. polium sharply decreased the potency of motility and cell invasion by the change in the expression of catenins and E-cadherin (23). Besides, aqueous and methanolic extracts of T. polium exerted cytotoxic effects on some cancerous cell lines, including a new type of glioblastoma multiforme (21, 24). Evidence (25, 26) demonstrated that known doses of T. polium had positive effects on learning and memory in diabetic rats. In addition, it could protect the hippocampus against neuronal damage.
Flavonoids are a class of compounds in this plant, which can trigger apoptosis through the activation of p53 in cells (24); the point well approved in the brain tissue in the present study. Teucrium polium can convert β-catenin, as a transcriptional regulator, to a cell-cell adhesion molecule by inhibiting the phosphorylation of β-catenin via Src dephosphorylation. Accordingly, the inhibition of cell cycle progression, cellular degeneration, apoptosis, and cell death, induced by T. polium extracts might be associated with the two mentioned paths (24).
In addition to the neurotoxic activity of T. polium, the effect of long-term administration of a sub-lethal dose (126 mg/kg) of T. polium extracts was also investigated on the kidney and liver. The animals treated with the extract and fractions of T. polium displayed significant destruction in the liver structure. However, the PE fraction of T. polium increased apoptosis and formation of small vacuoles and ballooned hepatocytes in the liver tissue compared with T. polium crude extract and DE fraction. These findings were in agreement with those of some studies reporting adverse effects of T. polium on the liver tissue (27-29). Nevertheless, some papers reported its protective role in liver cells (30, 31). Panovska et al. (30), demonstrated that the ethyl acetate extract of T. polium had hepatoprotective effects on liver injury induced by carbon tetrachloride (CCL4). Administration of T. polium for seven days resulted in an enhanced level of liver superoxide dismutase (SOD) and glutathione peroxidase (GSH), along with a decrease in the level of thiobarbituric acid reactive substances. Also, pre-treatment of rats with CCL4 and T. polium extract improved the liver architecture. They believed that protective effects of T. polium were associated with prevention from GSH level reduction and free radicals scavenging. A study investigating the effects of T. polium ethanolic extract on the Fe2+/ascorbate toxicity model in liver homogenates, indicated that T. polium had an effective role in protection against oxidative effects induced by the Fe2+/ascorbate model. These protective effects are suggested to be due to the scavenging of reactive oxygen species (ROS) and other free radicals (32). Conversely, chemical studies on T. polium extracts showed that neoclerodane diterpenoids had toxic properties. The toxicity of neoclerodane diterpenoids is markedly documented. It was shown that teucrin A and teuchamaedryn A could induce abnormalities and even led to liver diseases. Cytochrome P450 3A4 (CYP3A4) can oxidize the furan ring of teucrin A and teuchamaedryn A. This transformation makes the reactive epoxide bind with proteins, such as epoxide hydrolase and CYP3A. An increase in the reactive epoxide results in mitochondrial permeability transition, caspase 3 and 8 activities, and eventually apoptosis in cells. Besides, teucrin A can covalently link to cell proteins and reduce cytoskeleton-associated protein thiols and cellular GSH level, but it increases cytosolic levels of Ca2+ ions. These events induce cytotoxicity, hypersensitivity reactions, oncogene activation, and even impair cellular integrity (33-35).
Moreover, the study findings described adverse histopathological effects of T. polium on the kidney tissue following six weeks of treatment. The severity of injuries induced by T. polium led to atrophy, vacuolization, and necrosis in the renal cells, although the DE fraction had the most cytotoxic agent. In line with the present study findings, Rafieian-Kopaei et al. (14), reported similar injuries in the renal tissue 28 days after the administration period. These findings were also confirmed by other researchers (35-37).
Although a majority of consumers believe that medicinal herbs are safe, it was observed that T. polium could cause problems due to its toxicity in long-term use. To the best of the authors’ knowledge, there was no report of the short-term toxicity of T. polium in the literature. Also, in traditional medicine, short-term or intermittent administration of T. polium is recommended, and there is a saying in this province that “longer use (more than one week) of T. polium makes you icteric”. However, further studies are required to show whether intermittent use of T. polium could be a solution against its cell toxicity or not. In addition, accumulating reports show that T. polium can act as a potential anticancer medicinal plant against various cancerous cell lines due to its powerful cytotoxicity, especially the PE fraction (24, 38, 39). A balance between its anticancer activity and its neurotoxicity and hepatotoxicity properties should be considered upon recommendation. Both safety and efficacy are generally the major keys in medicinal plants and medicine. These features come back to the toxicity of herbal medicine.
5.1. Conclusions
The findings indicated that at sub-lethal doses and long-term administration, crude extract and different fractions of T. polium are toxic and impair cellular integrity in the liver, brain, and kidney. At the cellular level, T. polium induces edema, atrophy, vacuolization, apoptosis, and necrosis in the tissue. Accordingly, T. polium should carefully be prescribed if it is administered for long periods. Also, further studies are required to understand the molecular mechanisms involved in the cytotoxicity of T. polium in various organs.