1. Background
Chronic pain persists for more than three to six months and is usually followed by chronic diseases or malignancies (1). The number of people diagnosed with chronic pain is increasing every year (2-4). Many patients with chronic pain complained about the ineffectiveness of their pain killer medication. It remains under debate whether some analgesics, such as morphine and adjuvant analgesic, are effective treatments of chronic pain (3). Hence, the discovery of new substances or methods for treating chronic pain is essential.
Intense exposure to chronic inflammation or neuropathy is pathophysiologically involved in chronic pain (5, 6). Specific pathways or mechanisms of chronic pain are complicated and unclear, making chronic pain challenging to cure. The inflammation process generally induces the release of inflammatory mediators such as prostaglandin (PGE2) via the activation of the cyclooxygenase-2 (COX-2) enzyme (7). Increased PGE2 levels activate nociceptors and stimulate signal transmission from peripheral nerves to the central nervous system through the release of glutamate, a key excitatory neurotransmitter in chronic pain. The binding of glutamate to its receptor, especially the N-methyl-D-aspartate receptor (NMDAR), is related to the sensitization of pain as hyperalgesia and allodynia (8-10). The NMDAR is composed of two subunits: NR1 and NR2A-NR2D (11). NMDAR2B phosphorylation is considered responsible for chronic pain pathways (12, 13). Increased NMDAR2B was reported in the dorsal horn of the spinal cords of mice with neuropathy (14). Moreover, a noxious stimulus changed the expression of both NR2A and NR2B subunits (12). Thus, NMDAR and COX-2 are good potential targets for new substances to relieve chronic pain.
In traditional medicine, red ginger oil (RGO) has been commonly used to treat many symptoms (15-17). Our previous study showed the effect of RGO administration in reducing hyperalgesia in a mouse model of chronic pain, which is induced by inflammatory or neuropathic means (17, 18). However, how RGO minimizes pain behavior remains unclear. Therefore, the present study aimed to observe the effect of RGO in inflammatory- or neuropathy-induced chronic pain with two main targets: COX-2 and NMDAR. To our knowledge, this study is the first report on how RGO changes COX-2 and NMDAR expression during chronic pain.
2. Objectives
The aim of this research was to examine the effect of RGO on cyclooxygenase (COX)-2 and the N-methyl-D-aspartate receptor (NMDAR) using inflammatory- or neuropathy-induced chronic pain in mice.
3. Methods
3.1. Materials
RGO was harvested and provided by organic farmer groups in Kencong, Jember, East Java, Indonesia. The material that was used in this research included complete Freund’s adjuvant (CFA; Sigma), normal saline (Otsuka), suture 8/0 (Ethicon), silk suture 3/0 (One Med), and syringe 26G (Terumo).
3.2. Animals
Male BALB/c mice (8 weeks; 20 - 30 g; n = 48) were provided by the Animal Housing Laboratory, Faculty of Pharmacy, University of Jember, East Java, Indonesia. All mice were housed 4 - 5 animals per 20 × 30 cm cage. The temperature was maintained at 26°C with a 12-h dark and light cycle to protect them from stress. Mice were fed a standard diet and given water ad libitum. Before the test, all mice were adapted for one week to minimize stress and bias.
3.3. Experimental Scheme
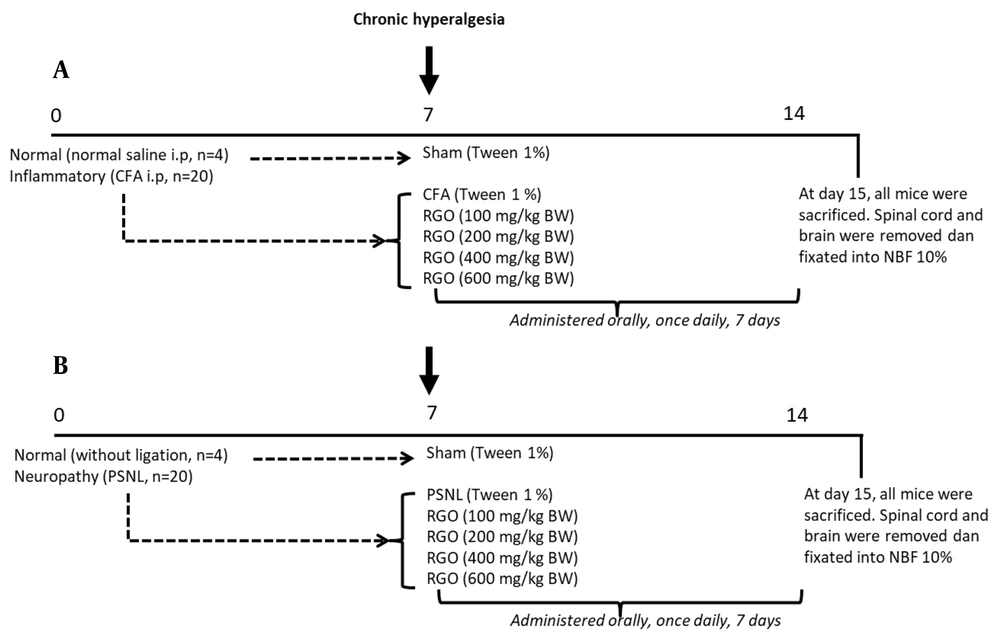
After adaptation for one week, mice were divided into two groups: the inflammatory and neuropathy models. The experimental design for each model is shown in Figure 1. To induce a chronic pain state, mice were conditioned until day 7 and characterized by prolonging hyperalgesia. Subsequently, mice were randomly divided further into treatment groups, and treatments were orally given, once daily, for seven days until day 14, as reported in our previous study (18). At the end of the study (day 15), all mice were sacrificed, and the spinal cords and brains were quickly removed and immediately fixed in 10% neutral buffered formalin for further tests.
Experimental scheme. Mice were divided into two groups: inflammatory, n = 24 (A) and neuropathy, n = 24. (B) models. To induce a chronic pain state, mice were adapted until day 7 and characterized by prolonging hyperalgesia. Mice were randomly divided further into treatment groups, and treatments were orally given, once daily, for seven days until day 14. Numbers (0, 7, 14) indicate days of the experiment. CFA, complete Freund’s adjuvant; i.p., intraperitoneal; NBF, neutral buffered formalin; PSNL, partial sciatic nerve ligation; RGO, red ginger oil.
3.4. Red Ginger Oil Distillation
Fresh red ginger was distilled at a composition of 1:2. This process was completed for 5 - 6 h at 100°C - 121°C. RGO was stored in a dark bottle away from direct light. The yield was 0.43%, as mentioned in our previous research (18).
3.5. Acute Toxicity Test
The acute toxicity test was evaluated using the Organization for Economic Co-operation and Development (OECD) guideline 423 on BALB/c mice. Before the trial, all mice were fasted overnight (12 h - 16 h) with free access to water. Mice were divided into two groups with three animals of each gender and received RGO 2,000 or 5,000 mg/kg. Mice were observed for any toxic effect for 4 and 24 h after RGO administration. After 24 h, the number of dead mice was counted. Mice were observed until day 14 to evaluate any delayed effect of the substance.
3.6. Complete Freund’s Adjuvant-Induced Chronic Pain
Mice were randomly divided into two groups: the sham (n = 4) and inflammatory (n = 20) groups. Before injection, mice were anesthetized with ether. The inflammatory group received 40 µL CFA intraplantar injection into the left hind paw. The sham group received the same volume of normal saline. Chronic inflammation followed by hyperalgesia was achieved after seven days (18).
3.7. Partial Sciatic Nerve Ligation-Induced Chronic Pain
Mice were randomly divided into two groups: the sham (n = 4) and neuropathic (n = 20) groups. Before the procedure, mice were anesthetized with ether. In the neuropathic group, one-third to one-half of the dorsal horn of the sciatic nerve on the left lumbar was tightly tied using 8 - 0 suture. The sham group underwent the same operation, but with no ligation. Neuropathy followed by hyperalgesia was achieved after seven days (18).
3.8. Hyperalgesia Hot-Plate Test
The hot-plate test was used to examine chronic hyperalgesia, per a previous study (18). Briefly, a hot plate (cat. 35100; Ugo Basile, Italy) was set at 50°C to activate C nerve fibers. After adaptation for 30 minutes, each mouse was individually tested on the hot plate. Pain behavior was visually observed and described as a lick, jump, or rear, whichever came first. The latency time toward a thermal stimulus was recorded as the time until the mouse first demonstrated a pain behavior. The duration of each test was limited to 30 minutes to prevent nerve damage. Hyperalgesia measurements were taken on day 0 (baseline, before induction) and days 1, 3, 5, and 7 (after induction). Mice with a baseline of fewer than 8 s were excluded from this study (18).
3.9. Histologic Analysis
On day 15 after induction, mice were anesthetized with ether, and the spinal cords and brains were quickly removed and fixed in 10% neutral buffered formalin. Specimens were transversely sectioned (3 - 4 µm) with a microtome. Spinal cord samples were stained with hematoxylin and eosin. The morphology of the dorsal horn of the spinal cord was observed using a light microscope (Olympus BX53) at 100× and 1,000× magnification. The changes in the dorsal horn’s morphology included hemorrhage, vacuolation, and degenerating neuron, glial nuclei, and neuronal nuclei (19).
3.10. Immunohistochemistry
Labeled-streptavidin biotin II was used for immunohistochemical analysis. Spinal cord samples were routinely processed using anti-mouse COX-2 (1:100; cat. bs-10411R; Bioss Antibodies, Woburn, MA, USA), anti-NMDAR2A (1:100; cat. bs-3507R; Bioss), and anti-NMDAR2B (1:100; cat. bs-0222R; Bioss) antibodies. Brain samples were analyzed using anti-mouse COX-2 (1:100; cat. bs-10411R; Bioss) antibody. COX-2, NMDAR2A, and NMDAR2B expressions in the dorsal horn of the spinal cord and hypothalamus of the brain were observed using a light microscope with 1,000× magnification.
3.11. Data Analysis
All data are described as mean ± standard deviation (SD). The differences between groups were analyzed using one-way ANOVA with Tukey’s post hoc test if the data was normal distributed and homogenous. Otherwise, Kruskall Wallis and followed by Mann Whitney analysis were used when the data did not fulfill the requirements. Data analysis was performed with GraphPad Prism 5 (San Diego, CA, USA). Significant differences between groups were interpreted if P-value < 0.05.
4. Results
4.1. Acute Toxicity Test
Mice injected with 2,000 or 5,000 mg/kg of RGO showed no toxic behaviors after 4 h, and all of them survived until 24 h. Even at day 14, all of the mice were in good condition, regardless of the dose. Thus, the LD50 value of RGO was less than 5,000 mg/kg and classified as safe or not acutely toxic, per Globally Harmonized System classifications.
4.2. Chronic Hyperalgesia Measurement
Before the induction of chronic pain, the CFA, PSNL, and sham groups showed the same hyperalgesia latency time by hot-plate assay. Therefore, all mice were in the same condition before induction. After induction with CFA or PSNL, mice experienced hyperalgesia, shown as continuously decreasing latency times until day 7, when these times became significantly different compared with those of the sham group (P < 0.05), consistent with the findings of our previous study (18). Thus, it was confirmed that mice were in a chronic hyperalgesia state.
4.3. Spinal Cord Histology
4.3.1. Histopathology of the Spinal Cords of Mice after CFA- or PSNL-Induced Chronic Pain
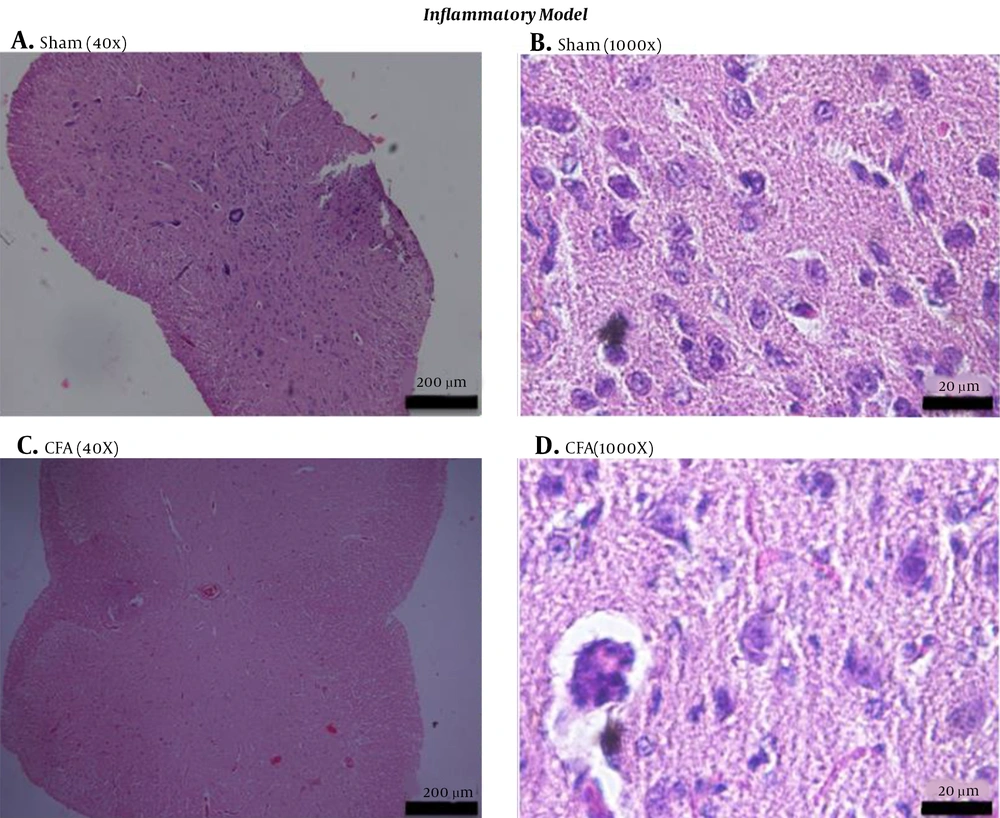
A suspension of Mycobacterium spp. comprises CFA. Intraplantar injection of CFA in mice caused chronic inflammation. The histology of the spinal cords from the CFA group was markedly different from that of the sham group. The CFA group contained a greater number of degenerating neurons (n), vacuolization (v), and inflammatory cells (i) than the sham control group (Figure 2). Inflammation can be characterized by the release of inflammatory mediators such as PGE2, increased membrane permeability, and vasodilatation. This condition leads to an increase in vacuolization and leukocyte infiltration, demonstrating a rising number of inflammatory cells. The inflammation process also induced neuronal damage, as demonstrated by the degeneration of neurons and myelin.
Histology of the spinal cords of mice in the inflammatory model of chronic pain. The spinal cords of sham (A, B) and CFA (C, D) groups of mice were harvested at day 15 after induction, fixed, sectioned, and stained with hematoxylin and eosin. Sections are viewed at 100× (A, C) or 1,000× magnification (B, D) under bright-field microscopy. CFA, complete Freund’s adjuvant.
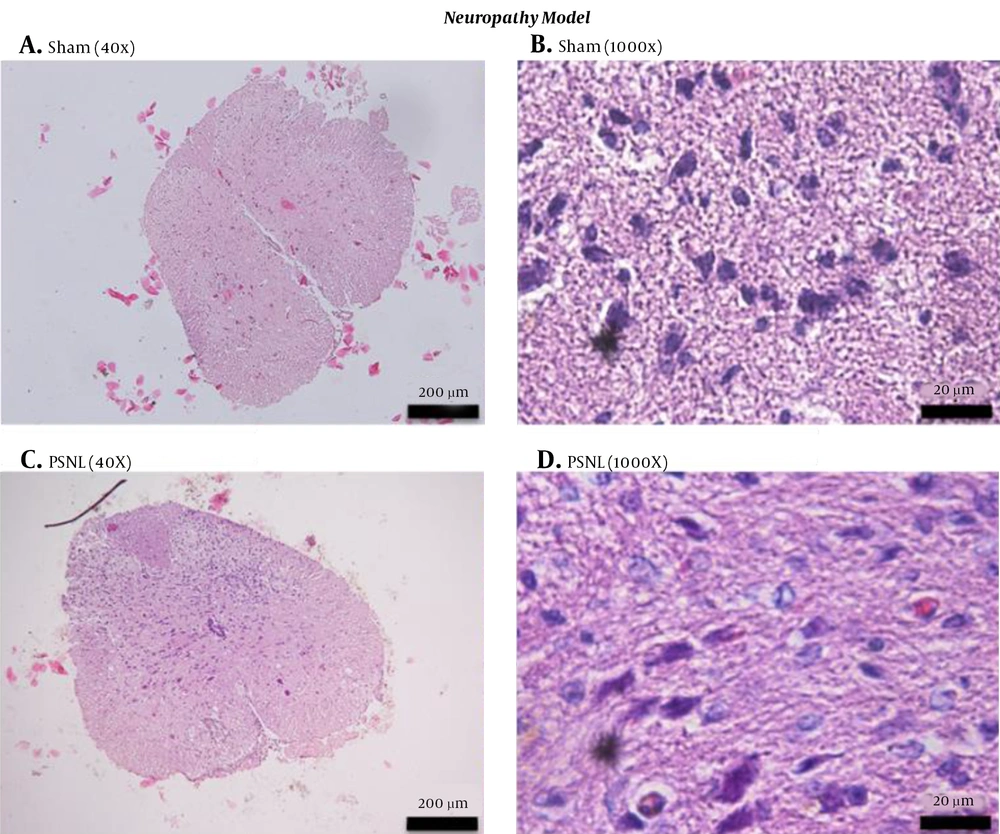
The direct damage of neurons because of trauma in neuropathy also changes the histology of the spinal cord of mice. We observed an increased number of degenerating neurons in the PSNL-treated group than in the sham group (Figure 3). PSNL group neurons also were in a protrusion state due to the activation of neurotransmitters involved in chronic pain, such as glutamate.
Histology of the spinal cords of mice in the neuropathy model of chronic pain. The spinal cords of sham (A, B) and PSNL (C, D) groups were harvested at day 15 after induction, fixed, sectioned, and stained with hematoxylin and eosin. Sections were viewed under 100× (A, C) or 1,000× magnification (B, D) under bright-field microscopy. PSNL, partial sciatic nerve ligation.
4.3.2. Spinal Cord Histology after RGO Treatment
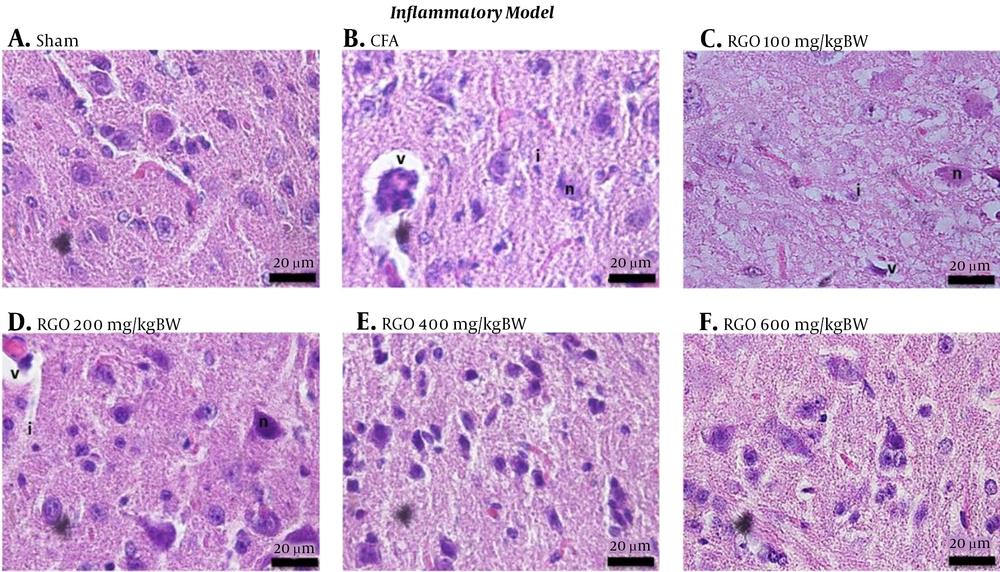
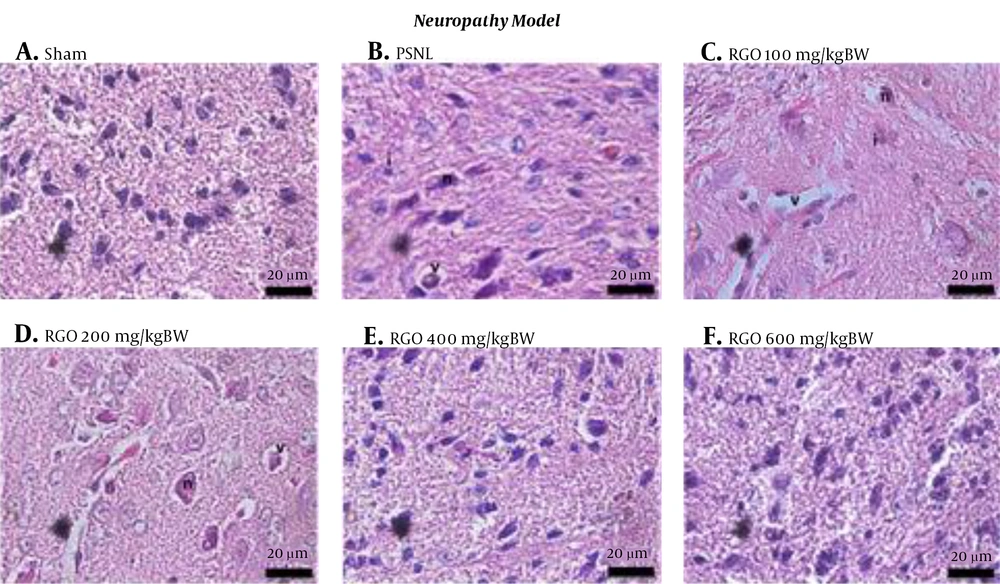
After RGO administration, spinal cord morphology was improved in both inflammatory and neuropathy models, with restoration close to that of the sham group. The decreasing amount of vacuolization, inflammatory cell infiltration, and degenerating neurons were linearly correlated with the increasing dose (Figures 4 and 5). Thus, RGO at 600 mg/kg provided the best improvement. These findings are supported by our previous study demonstrating that RGO at 600 mg/kg adequately minimized hyperalgesia in mice with chronic pain induced by CFA and PSNL (18).
Histology of the spinal cords of mice under different treatment conditions in the inflammatory model of chronic pain. A, Sham; B, CFA; C, RGO 100; D, RGO 200; E, RGO 400; and F, RGO 600 mg/kg body weight (BW) are shown. Hematoxylin and eosin staining, 1,000× magnification. Labels: v, vacuolization; n, degenerating neuron; i, inflammatory cells. CFA, complete Freund’s adjuvant; RGO, red ginger oil.
Histology of spinal cords of mice under different conditions in the neuropathy model of chronic pain. A, Sham; B, PSNL; C, RGO 100; D, RGO 200; E, RGO 400; and F, RGO 600 mg/kg body weight (BW) treatments are shown. Hematoxylin and eosin staining, 1,000× magnification. Labels: v, vacuolization; n, degenerating neuron; i, inflammatory cells. PSNL, partial sciatic nerve ligation; RGO, red ginger oil.
4.4. Expression of COX-2 and NMDA Subunits NR2A and NR2B Using Immunohistochemistry
4.4.1. Expression of COX-2 and NMDA Subunit NR2B in CFA-Induced Chronic Pain After Treatments
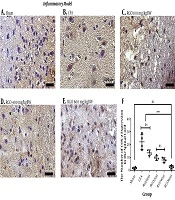
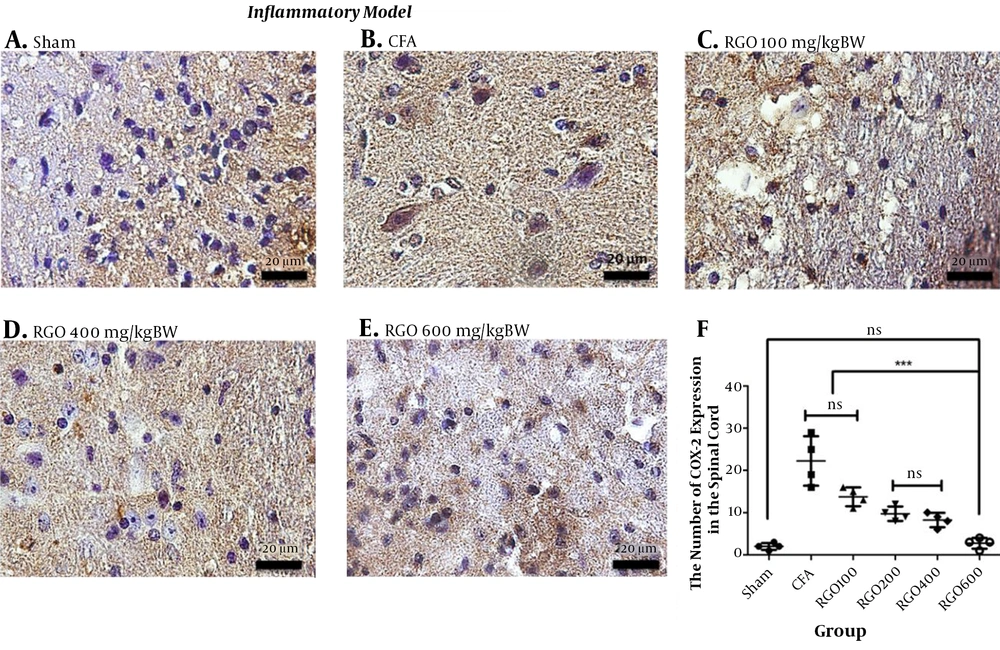
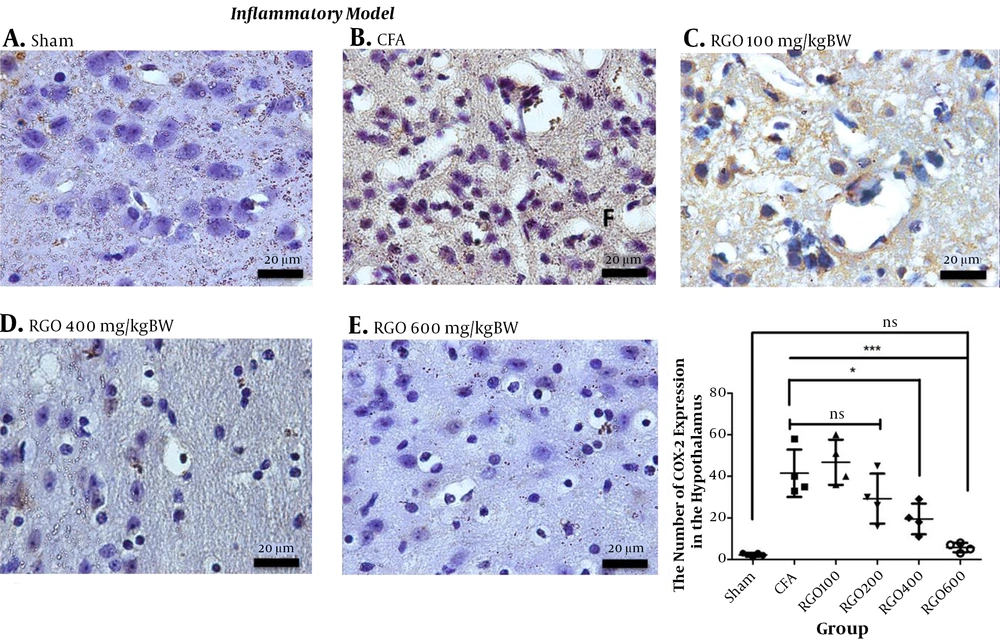
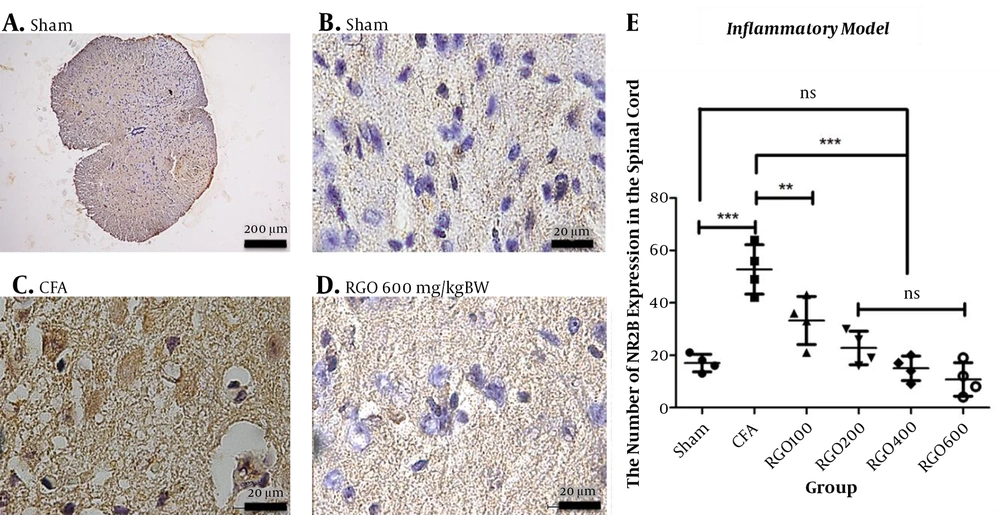
RGO administration reduced COX-2 expression after inflammatory pain compared with the CFA group without RGO. The highest dose of RGO (600 mg/kg) inhibited COX-2 expression to the same levels as the sham group (P > 0.05; Figure 6). The decreased amount of COX-2 expression in the spinal cord (Figure 6) impacted COX-2 expression in the hypothalamus. Robust noxious stimulus in chronic pain caused peripheral sensitization to become central sensitization as well. Furthermore, RGO suppressed COX-2 expression in the hypothalamus. RGO at a dose of 600 mg/kg showed the best activity in reducing COX-2 expression in the hypothalamus to a level not significantly different from the sham group (P > 0.05; Figure 7).
Immunohistochemical analysis of COX-2 expression in the spinal cord of mice in the inflammatory model of chronic pain. A, Sham; B, CFA; C, RGO 100; D, RGO 400; and E, RGO 600 mg/kg body weight (BW) treatments are shown; F, The number of COX-2-positive cells in the spinal cord (number ± SD; n = 4). Magnification, 1,000×; ns, not significantly different (P > 0.05). Significant differences (***, P < 0.001) were determined by one-way ANOVA with Tukey’s post hoc analysis. Linear regression s r2 value = 0.7368. CFA, complete Freund’s adjuvant; RGO, red ginger oil.
Immunohistochemical analysis of COX-2 expression in the hypothalamus of mice in the inflammatory model of chronic pain. A, Sham; B, CFA; C, RGO 100; D, RGO 400; and E, RGO 600 mg/kg body weight (BW) treatments are shown; F, The number of COX-2-positive cells in the brain (number ± SD; n = 4). Magnification, 1,000×; ns, not significantly different (P > 0.05). Significant differences (*, P < 0.05 and ***, P < 0.001) were determined via one-way ANOVA with Tukey’s post hoc analysis. Linear regression between dose and COX-2 expression r2 value = 0.7040. CFA, complete Freund’s adjuvant; RGO, red ginger oil.
Our prior study demonstrated that RGO administration improved pain behavior such as hyperalgesia after CFA-induced chronic pain. In this study, we discovered that RGO also affects the expression of NMDAR2B, the primary receptor in the chronic pain pathway. CFA injection significantly stimulated NMDAR2B expression in the spinal cord over that of the sham group (P < 0.001; Figure 8). However, the oral administration of RGO for seven consecutive days inhibited this NMDAR2B overexpression in the spinal cord. Different from the COX-2 result, RGO doses above 100 mg/kg BW were not significantly different from each other or the sham group (P > 0.05; Figure 8).
Immunohistochemical analysis of the expression of NMDA subunit NR2B expression in the spinal cords of mice in the inflammatory model of chronic pain. A, Sham (100× magnification); B, Sham (1,000×); C, CFA (1000×); and D, RGO 600 mg/kg body weight (BW) (1000×) are shown; E, NMDAR2B expression in spinal cord neurons (number ± SD; n = 4 mice). Statistical analyses: ns, not significant (P > 0.05); **, P < 0.01; ***, P < 0.001 by one-way ANOVA with Tukey’s test. Linear regression between dose and NMDAR2B expression r2 value = 0.6920. CFA, complete Freund’s adjuvant; RGO, red ginger oil.
4.4.2. Expression of NMDA Subunit NR2A and NR2B in PSNL-Induced Chronic Pain After Treatments
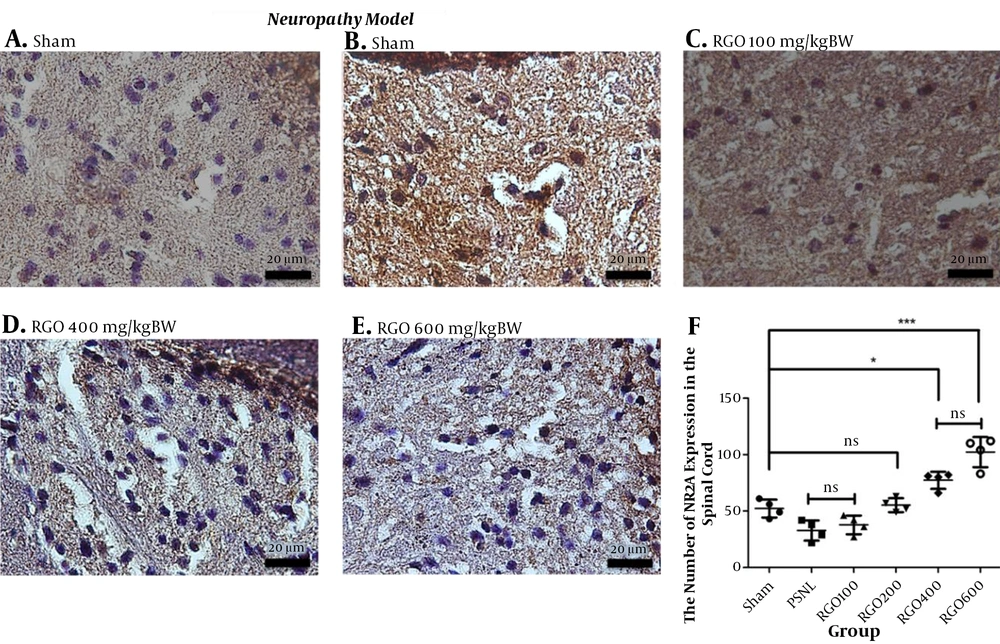
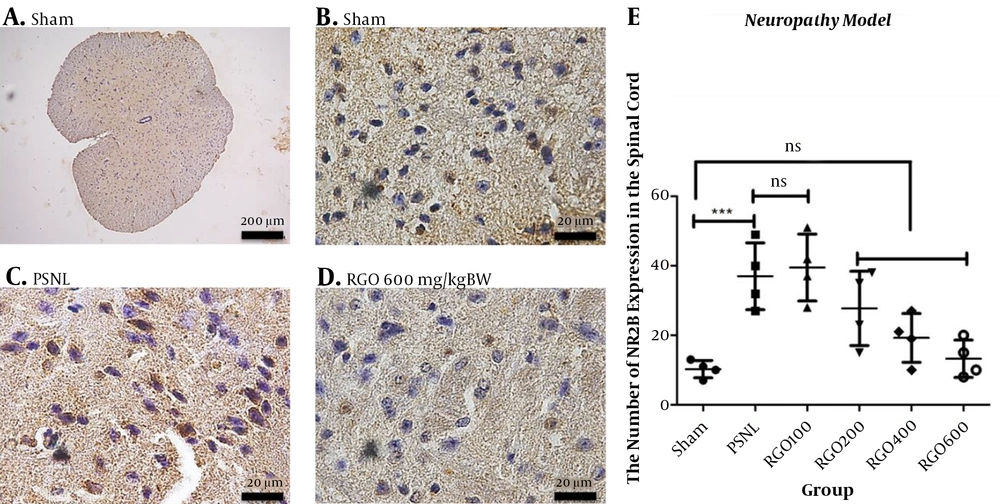
The direct damage of neurons through neuropathy changed the composition between NMDA subunit NR2A and NR2B receptors in the spinal cord. After the PSNL method, the expression of the NR2A subunit decreased and the NR2B subunit increased. Treatment with RGO significantly increased NMDAR2A expression and diminished NMDAR2B expression in the spinal cord (Figures 9 and 10).
Immunohistochemical analysis of the expression of NMDA subunit NR2A in the spinal cords of mice in the neuropathy model of chronic pain. A, Sham; B, PSNL; C, RGO 100; D, RGO 400; and E, RGO 600 mg/kg body weight (BW) are shown. F: NMDAR2A expression in spinal cord neurons (number ± SD; n = 4 mice). Statistical analyses: ns, not significant (P > 0.05); *, P < 0.05; ***, P < 0.001 by Kruskall Wallis followed by Mann Whitney analysis. Linear regression between dose and NMDAR2A expression r2 value = 0.9062. PSNL, partial sciatic nerve ligation; RGO, red ginger oil.
Immunohistochemical analysis of the expression of NMDA subunit NR2B in the spinal cords of mice in the neuropathy model of chronic pain. A, Sham at 100× magnification; B, Sham at 1,000× magnification; C, PSNL (1000× magnification); D, RGO 600 mg/kg body weight (BW) (1,000× magnification) are shown; E, NMDAR2B expression in spinal cord neurons (number ± SD; n = 4 mice). ns, not significantly different (P > 0.05); ***, P < 0.001 by one-way ANOVA followed by Tukey’s test. Linear regression between dose and NMDAR2B expression r2 value = 0.5917. PSNL, partial sciatic nerve ligation; RGO, red ginger oil.
5. Discussion
Chronic inflammation and neuropathy are two of the leading causes related to chronic pain incidence (5). Persistent inflammation is believed to play a role in the occurrence of neuropathy. The release of prostaglandins, chemokines, and cytokines after chronic inflammation induces neuronal damage and leads to neuropathy. This indirect action also promotes central sensitization, as well as direct neuropathy (20, 21).
The evaluation of pain using animal models can be questionable. Based on many protocols, thermal hyperalgesia using a hot plate is one method that has been thoroughly validated (22-24). Chronic pain conditions in animals could be defined as prolonged hyperalgesia shown as a shortening of the latency time toward the thermal stimulus (22, 23). In our study, hyperalgesia occurred until seven days after the induction of inflammation by CFA or neuropathy by PSNL. Several recent reviews (18, 25-27) corroborate the same trends described in this study.
The intraplantar CFA injection successfully activated phospholipid membranes to release COX-2 and produced PGE2. Such chronic stimuli not only induce PGE2 but also trigger the action of chemokines and cytokines (20, 28). This condition allows sodium ion influx, causing depolarization (8). Calcium influx into the cell after depolarization initiates the release of glutamate, which binds to its receptor NMDAR2B, which is associated with chronic pain behavior, including hyperalgesia (5, 11). One study explained that chemokine (C-X-C motif) ligand 1 (CXCL1) release in the inflammatory pain pathway increases COX-2 expression and NMDA subunit NR2B activation via chemokine receptor 2 (CXCR2) activation (29). The appearance of COX-2 usually occurs in peripheral nerves. Here, we demonstrated the increase of COX-2 expression in nerves of the spinal cord and brain after CFA induction. COX-2 is known to mediate chronic inflammatory responses; RGO administration reduced COX-2 expression in mouse spinal cords and brains and increased NMDAR2B expression in the spinal cords. These findings suggest a relationship between COX-2 and NMDAR2B, although prior research demonstrated an independent contribution by both of them. CFA is involved in several signaling cascades, such as protein kinase C γ and extracellular signal-regulated kinases (ERK)1/2, which may be NMDAR-dependent (30). The discovery of COX-2 expression in the spinal cord and brain also implies that RGO crosses the blood-brain-barrier and centrally acts.
PSNL, by contrast, causes direct injury to the sciatic nerves and also stimulates depolarization. Neuropathy also increases COX-2 expression, which is involved in depolarization. Prolonged depolarization, caused by both models used herein, triggers the overproduction of glutamate, leading to the loss of magnesium binding on NMDAR2B and activating this critical receptor in the chronic pain pathway. In our study, the direct damage of neurons because of trauma in neuropathy seems also change the histology of the spinal cord of mice as showed as the increasing expression of NMDAR2B in the dorsal horn area of spinal cord. RGO as a treatment suppressed NMDAR2B expression in the spinal cord but increased NMDA subunit NR2A expression in the spinal cord. Many previous studies have demonstrated the involvement of NMDAR2B in neuropathy-induced chronic pain pathophysiology (14, 31-34).
Our study suggests a relationship between COX-2, NMDAR2A, and NMDAR2B. The suppression of COX-2 expression caused inhibition of PGE2 release and prevented membrane depolarization. A low membrane potential would inhibit the release of pain neurotransmitters, such as glutamate, decreasing the interaction between glutamate and its receptor NMDAR2B (9, 11). The composition between NMDA subunits NR2A and NR2B is essential in the synaptogenesis process, especially in chronic pain. In chronic pain pathophysiology, NMDAR2B was related to pain sensitization (11). Decreasing NMDAR2B has been indicated in reducing hyperalgesia and allodynia in chronic pain. NMDAR2A is less sensitive to glutamate than NMDAR2B. Thus, NMDAR2A plays a larger role than NMDAR2B in synapse stabilization and neuron maturation, related to neuron regeneration (12). Interestingly, decreased NMDAR2B expression and increased NMDAR2A are involved in dendritic branch development (34). NMDAR2A also plays an important role in neuron regeneration. NR2A activation successfully induces the phosphorylation of cAMP response element-binding protein, which is associated with brain-derived neurotrophic factor expression and contributes to neuronal survival that might be important for neuroprotective activity (35).
This study was the first report on the effect of RGO in COX and NMDA expression in inflammatory- or neuropathy-induced chronic pain in mice. This result also supported our previous work that RGO reduced hyperalgesia in mice after induced by CFA and PSNL. Other studies about the activity of RGO in chronic pain were very limited. Some of the data were revealed the effect of ginger oil, not red ginger oil. Our best finding was Funk et al. (36), which studied the effect of ginger oil in rheumatoid arthritis, and Jia et al. (37), who reported ginger oil activity in reducing hyperalgesia in CFA-induced chronic pain.
Essential oils are the main compound of RGO. Our latest study demonstrates that RGO is composed of several essential oils, such as camphene, cineol, borneol, citronellol, linalool, and myrcene (18). Many studies have revealed the antihyperalgesic activity of several essential oils (38-41). Although the precise mechanism remains unclear, the essential oil may inhibit COX-2, transient receptor potential vanilloid 1, or cytokines (39, 40). The information about how the essential oil could pass through the brain is still very lack. According to the barrier of brain characteristic, essential oils which are lipophilic could easily across the blood-brain barrier then bound the pain receptor. However, this statement needs more clarification and further research.
RGO administration caused an improvement in the morphology of spinal cord’s mice with chronic pain. However, this study did not examine the exact mechanism related to its effect, as our limitation. Our research also revealed RGO at 600 mg/kg demonstrated the best activity in reducing COX-2 and NMDAR2B expression in mice’s spinal cords with chronic pain. The role of NMDAR2A in chronic pain remains unclear. Another limitation in our research was the expression of NMDAR2A not demonstrated in both models, inflammatory and neuropathy pain. Therefore, the relationship between NMDA subunit NR2A and NR2B in the context of chronic pain requires further study.
5.1. Conclusions
RGO administration improved spinal cord mice’s morphology. RGO dose 600 mg/kg BW showed the best activity in inflammatory- and neuropathy-induced chronic pain by inhibiting COX-2 and NMDA pathways, particularly subunit NR2B. RGO administration also increased NMDAR2A, and the role of NMDAR2A remains unclear and needs further investigation.