1. Background
Wound healing is significant in terms of confirming exponential veracity of the skin and its defensive and efficient arrangement, which occurs with the patch-up of the skin (1). Inflammatory reaction, cellular reproduction, and skin formation are considered as the main steps for healing wounds. Some researchers believe that finding a new method incorporating all of these steps is a big step towards wound healing (2-4). Nowadays, leech therapy is being used for treating different kinds of health problems around the world.
The prompt achievement of using leech in malleable and restoring normal function surgery, as well as handling degenerative and diseases-related long-lasting pain conditions caused huge approval in current treatment. Another success for using medicinal leeches is related to its acceptance by the US in 2004 as a medical device (5). Accordingly, Avicenna, the great Ancient Iranian scientists, in his book entitled “Canon of Medicine” and Seyyed Mohamad Hossein Aghili Khorasani-e Shirazi in his book entitled “Makhzan al Advieh” (6, 7) alluded using leech for treating different kinds of illnesses, among which wound healing is emphasized. Leeches have been used for medicinal purposes since circa 1500 B.C. However, a significant improvement has occurred in our knowledge about leech-related research over the last decade, leading to advancement in molecular techniques and genetic analyses (5). Leech salivary gland secretion (SGS) contains biologically active compounds which mainly consist of proteins and peptides, and have important biological activities in terms of treating several illnesses (8). Major bioactive constituents of leech include tryptase inhibitor (9), Bdellastasin (bdellin A), Hirudin (10), Saratin (11), destabilase lysozyme (12), hyaluronidase (13), calin (14), glutamyl transpeptidase (15).
The major part of tentative medium in skin wound is formed by fibronectin and fibrin, which plays a part of healing development. Fibronectin is predicted to be a valuable part in curing skin wounds. Fibronectin decreases in the wounds taking a long time for their repairment. The reduction of fibronectin in long-lasting wound fluid enzymes was repressed by α1-antitrypsin. The amount of fibronectin increases by increasing the amount of α1-antitrypsin. Based on the results, there are enzymes in chronic wounds which discompose the role of α1-antitrypsin and cause fibronectin deprivation by unrestrained serine proteinases (16). Leech SGS containing trypsin inhibitory activity may play an important role in a wound healing procedure. Factor Xa inhibitor and Hirudin block thrombin and inhibit plasma hemostasis (9) The anticoagulant effect of better blood flow ulcer area is expected to become available nutrients and oxygen, which results in accelerating the process of wound healing.
2. Objectives
The present study sought to prepare pharmaceutical skin product from lyophilized Hirudo orientalis leech source with active enzyme and proteins having wound healing and anti-coagulation activities. Furthermore, the quality control of the resultant product was analyzed by setting up the way for controlling its antitrypsin and anticoagulation activities.
3. Methods
3.1. Collecting and Identifying the Medicinal Leech
Hirudo orientalis was grown in the Bio-factory of the College of Science, University of Tehran. All leeches (n = 5 per 100 mL cream) weighing 0.9 - 1 g with 9-month age had not eaten any blood for 3 months. Leaches were starved for at least four months because this period of starvation is necessary for synthesis saliva content (8). Active leech ingredients were prepared from lyophilized leech and their antitrypsin and anticoagulation activities were measured by using Choudhary and Thomsen bioassay techniques and quagula meter (17).
Male Wistar rats (n = 30) considering 270 ± 30 g were purchased from Tehran University, Iran. The experimental rats were classified into five collections, each covering 6 rats. This animal investigation was based on National lnstitute of Health Guide in Laboratory Animals considerations (NIH publications no.: 80-23) and was confirmed by Ethics Committee of Tehran University, Iran. The rats were anesthetized using ketamine and xylazine mixture (80 and 100 mg/kg of body weight, respectively). The source of human blood (n = 6) was provided from department of blood bank - Pasteur Institute of Iran and the ethics approval consent was given according to the ethical code 9321427001.
3.2. Lyophilizing and Preparation of Biological Active Substance
Leeches were pulverized after lyophilization at -80°C for 48 hours. Pulverized leeches were mixed with 10 mL solution Sodium chloride 0.9% in deionized water and incubated for 45 min. Then, the sample was centrifuged at 15000 g for 30 min. Finally, the supernatant was used as the biological active substance (BAS) in the later stages of the study.
3.3. Trypsin Inhibition Assay
Trypsin inhibition assay was conducted to evaluate one of the salivary proteins of leech having antitrypsin effect. First, 121.14 g of hydroxylmethyl-aminomethne (Sigma) were melted in deionized water and the final volume of 1000 mL was prepared with pH = 7.5. In the next procedure, Substrate (1M Na-Benzoyl-L-arginine 4-nitroanilide; Sigma-Aldrich, St. Louis, Missouri, United States) and enzyme (trypsin; 2500U USP) were prepared as described previously (17). The overall chemical reaction is as follow:
Trypsin + Na-benzoyl-L-arginine 4-nitroanilide - hydrochloride (in the presence of Tris; pH = 7.5) → Nitroanilide hydrochloride
The yellow color of the resultant reaction product was analyzed by UV-Visible using a spectrophotometer device (Pharmacia ultraspec III LKB- Biochrom England) at a 410 nm wavelength. Our experimental layout of the whole chemical reaction for primary active ingredient from leech (PAIL) is as follows.
1) Trypsin + Na-benzoyl- L- arginine- 4- nitroanilide hydrochloride in Tris (pH = 7.5);
2) Trypsin + Na-benzoyl- L- arginine- 4- nitroanilide - hydrochloride + leech extract or BAS (10, 20, 30 and 40 µL);
3) Blank (10, 20, 30, 40 µL leech extract) + tris (pH = 7.5).
The whole chemical reaction for final product is described below.
1) Trypsin + Na-benzoyl- L- arginine- 4- nitroanilide hydrochloride in Tris (pH = 7.5);
2) Trypsin + Na-benzoyl- L- arginine- 4- nitroanilide hydrochloride + 5% leech cream (100, 200 and 300 mg);
3) Blank (100, 200, 300 mg of 5% leech cream) + tris (pH = 7.5).
3.4. Coagulation Assay in Leech Product
Coagulation activity assay was performed to study the effectiveness of anticoagulant proteins of SGS. First, 3.2 µL in 3.8% (w/v) sodium citrate (Sigma-Aldrich, St. Louis, Missouri, United States) was poured in each test tube, and 2.3 mL of fresh human blood was added to it. The whole mixture was centrifuged at 5000 × g for 20 min at 4°C and 5% leech cream (at the concentration of 0, 100, and 200 mg) was separately added to each test tube and rotated for 45 min. Then, prothrombin time (PT) and partial thromboplastin time (PTT) were analyzed by Coagulometer (Elite 9000) to observe the coagulation activity of leech product when the anticoagulant proteins in SGS such as Hirudin change the normal value of PT and PTT.
3.5. Formulation and Evaluation of 5% Leech
Regarding the base cream formulation, it was decided that the base cream should be Oil/water since the active ingredient is protein and soluble in water. The ingredients used for the cream formulation were Eucerin (3%), methyl paraben (0.02%), propyl paraben (0.02%), vaseline (20%) , propylenglycol (4%), cetylalcohol (4%), hard fat (4%), Tween 40 (0.15%), 80 (0.2%), BAS (5%), and distilled water (59.61%).
1) The amount of 100, 200, and 300 mg of 5% leech cream with the viscosity range of 65000 - 75000 cP were weighted and poured in the micro-tubes. Then, 1 mL of “solution A” (Tris buffer, pH = 7.5) and 500 µL of “solution B” (Urea 9M, 3-[(3-cholamidopropyl) dimethylammonio]-2-hydroxy-1-propanesulfonate 4%, triton 0.2%) was vortexed well and added to the mentioned micro-tube containing leech cream. The resultant solution was kept at room temperature overnight and its 500 µL was added to 1000 µL Na-benzoyl- L- arginine- 4- nitroanilide - hydrochloride, 500 µL trypsin at 37°C for 30 min, and this complex was analyzed spectrophotometrically at 410 nm.
2) leech cream (1g of 5% (w/v)) was poured in micro tube, 2000 µL solution A and 1000 µL of solution B were added, vortexed well, kept overnight at room temperature. For hirudin enzyme separation from leech product, 12% SDS-PAGE analysis was performed. The Hirudin band around 7 kDa was incised for elution procedure (18). To the eluted protein, 500 µL solution B was added (solution C). In another test tube, 3.2 µL of 3.8% sodium citrate, and 2.3 mL of fresh human blood were added to it and centrifuged at 5000 × g for 20 min at 4°C, then 80 µL of solution C was added to the resultant mixture, rotated for 45 min, and underwent PT and PTT analysis through coagulometer.
3.6. Franz Cell Analysis of Skin Absorption Capacity
Phosphate buffer pH = 7.5, stock A, 2.4 g NaH2PO4/100 mL ultra-pure water; stock B, 2.84 Na2HPO4/100 mL ultra-pure water, 27.7 A + 97.5 B + ultra-pure water (UPW) up to 250 mL, 1.5 gr 5% leech cream, 32 mL of buffer in cell at 37°C, and dialysis tubing 12 KD were prepared. The molecular weight of trypsin inhibitor enzyme in leech and Hirudin is 4.6 and 7 KD, respectively (19).
To evaluate skin absorption capacity and rheology, 5% leech cream was applied into the dialysis tubing of 12 KD diameter. The dialysis membrane was placed into the buffer filled tank at 37°C. The temperature of the experimental tank was adjusted to 37°C. After 12 h, the whole buffer was lyophilized and trypsinized to investigate whether the active leech ingredient could pass through the dialyzing membrane or not.
3.7. In Vivo Wound Healing Model
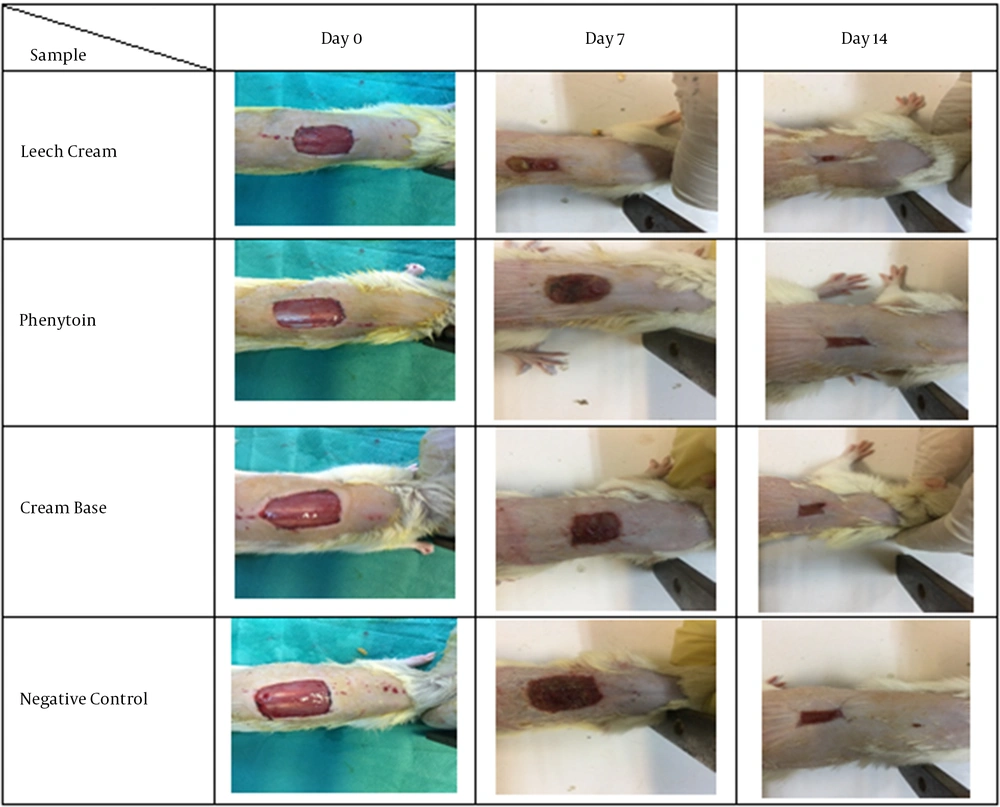
Rat model was used for in vivo evaluation of active leech product. The rats considered for experiment were separated into five collections, each holding 6 rats. The antral region of the rats was shaved and 1 × 1 cm2 wound was created by cutting the skin using a scalpel. The wounds were treated every other day by applying 1 g of the sample, phenytoin (positive control), or cream without active ingredient (negative control) to the injured sites. Sterile bandage was placed onto the treatment areas. Before each treatment, a photo was taken from the injured sites. Finally, skin samples were collected for histopathological analysis.
3.8. Histopathology of Wounds
Four animals from each group were euthanized every 7th and 14th day in healing management and the samples were proximately stabled and collected in the 10% formalin buffer (pH = 7.26) for 48 h. Then, the collected samples were fixed in paraffin, and divided into five µm thick slices. Finally, they were stained with haematoxylin and eosin (H & E). The prepared histological slides were evaluated by light Microscope (Ollympus BX51; Tokyo, Japan). Restoring skin stratums, corruptive cell penetration, and granular matter formati1on were evaluated in all treatment and control groups.
3.9. Histomorphometirc Assay
Restoring skin stratums at 14th day was assessed semi-quantitatively on five-point scale (O: without new repairing in skin, 1: 25%, 2: 50%, 3: 75%, and 4: 100%) in recovery percentage. Histological evaluation, angiogenesis, and collagen mass were performed with Image-Pro Plus® V.6 (Media Cybernetics, Inc., Silver Spring, USA). Kruskall Wallis test was used for data analysis.
3.10. Quality (Microbial) Control Test
The microbial test of leech cream on the nutrient medium in the laboratory was performed to ensure that there is no microbial contamination of the final product. Then, Mueller Hinton agar in petri dishes and serial dilutions of the sample in Ringer solution were prepared. In addition, 20 µL of each sample-dilution with different concentrations including 100, 10-1, 10-2, and 10-3 colony-forming unit (CFU/g) was administered onto the growth medium and incubation at 37°C for 48 h, and finally total colony count (TCC) was determined.
3.11. Statistical Analysis
All results were compared using Kruskal Wallis test in SPSS software, version 20.0. P < 0.05 was considered as statistically significant. All graphs were drawn with GraphPad Prism v6 software. The experiments were performed triplicate and were reported as mean ± SEM.
4. Results
4.1. In Vivo Wound Healing Model
Wound reduction indicated the ratio of enhancing restored region. An excision wound model was determined to study the mentioned model and subsequent epithelialization. Figure 1 displays the macroscopic changes of the wound area within 14 days. On the day 14, leech cream group showed the maximum percentage of wound reduction (80%) compared to Phenytoin group (15%), group treated with gel base (20%), and negative group (35%) (Figure 1).
4.2. Evaluation of Skin Absorption of Trypsin Inhibitory Substance by Franz Cell Method
Franz Cell analysis on lyophilized buffer solution proved that the active ingredient of 5% leech cream could pass through the dialysis membrane. Based on the results, absorption using Franz Cell method of 5% leech cream was significantly lower than zero concentration (t = 208.76, P < 0.0005).
4.3. Evaluation of Trypsin Inhibitor Activity
Final protein concentration of the 0, 100, 200 and 300 mg of 5% leech cream was calculated to 0, 166.930, 333.860, and 667.730 µg/µL, using Bradford method after 30-minute incubation at 37°C (20). Trypsin inhibitor activity of the mentioned protein concentration was 1.9, 1.47, 0.753, and 0.00 (Figure 2). Trypsin activity had a negative relationship with increasing concentrations of leech cream ranging from 0 to 300 mg/ml (P < 0.05; χ2 = 21.6).
Trypsin inhibitory effect of 5% leech cream, the concentration chart of 100, 200 and 300 mg of cream (horizontal) versus absorption at 410 nm (vertical), respectively (0.6, 1.2, 2.4). Trypsin activity had negative relationship with increasing concentrations of leech cream, ranging from 0 to 300 mg/ml. (Kruskal Wallis analysis, P < 0.05; χ2 = 21.6).
4.4. Anticoagulation Activity
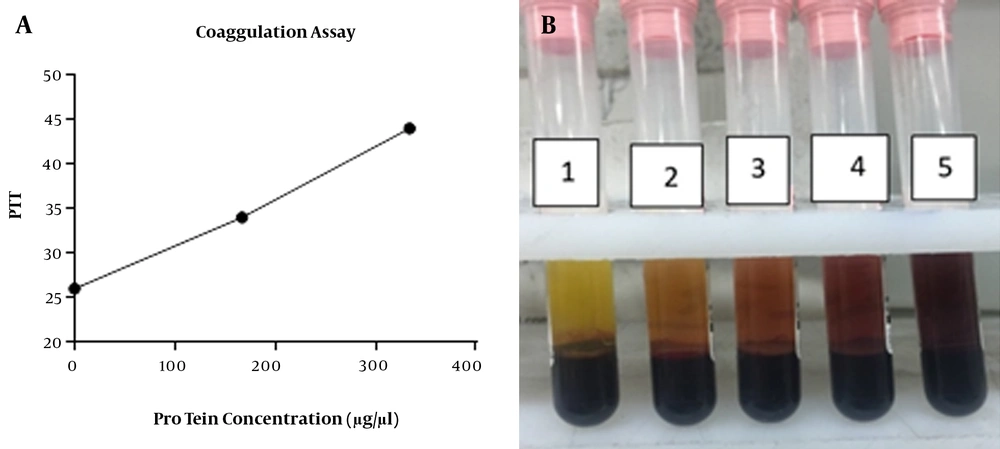
The results indicated that 0, 100, and 200 mg of 5% leech cream with protein concentration of 0, 166.930, and 333.860 µg/µL on 3.8% sodium citrate + 2.3 mL fresh blood had no effect on PT (prothrombin time), while could significantly influence PTT (partial thromboplastin time) (sec) (Mean ± SEM: 26 ± 0.00, 34.22 ± 0.61, and 42 ± 0.71) (Figures 3A and B).
Anti-coagulation activity of 5% leech cream, A, The concentration chart of 0, 100 and 200 mg of cream (horizontal) versus PTT (vertical), respectively 26, 34, 44. Leech cream has remarkable effect on PTT. Anti-coagulation activity had positive relationship with increasing concentrations; B, The photograph of anti-coagulation properties of lyophilized leech extract. The results showed that the following enhancement of the concentration of lyophilized leech extract, anti-coagulation properties have been increased. The number of tubes from left to right includes 10, 20, 40 and 80 µL of lyophilized leech extract into fresh human blood.
4.5. Coagulation Assessment by SDS PAGE
The 7 kDa region of Hirudin in SDS PACE gel electrophoresis was obtained by loading 5% leech cream onto gel after elution procedure as described above. This experiment was done in the presence of 0, 80, and 200 µL solution of purified protein, where the changes in PTT (sec) value were 26 ± 0.00, 39.6 ± 0.88, and > 70.
4.6. Wound Healing Capacity
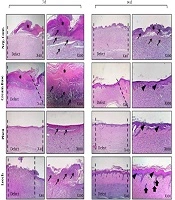
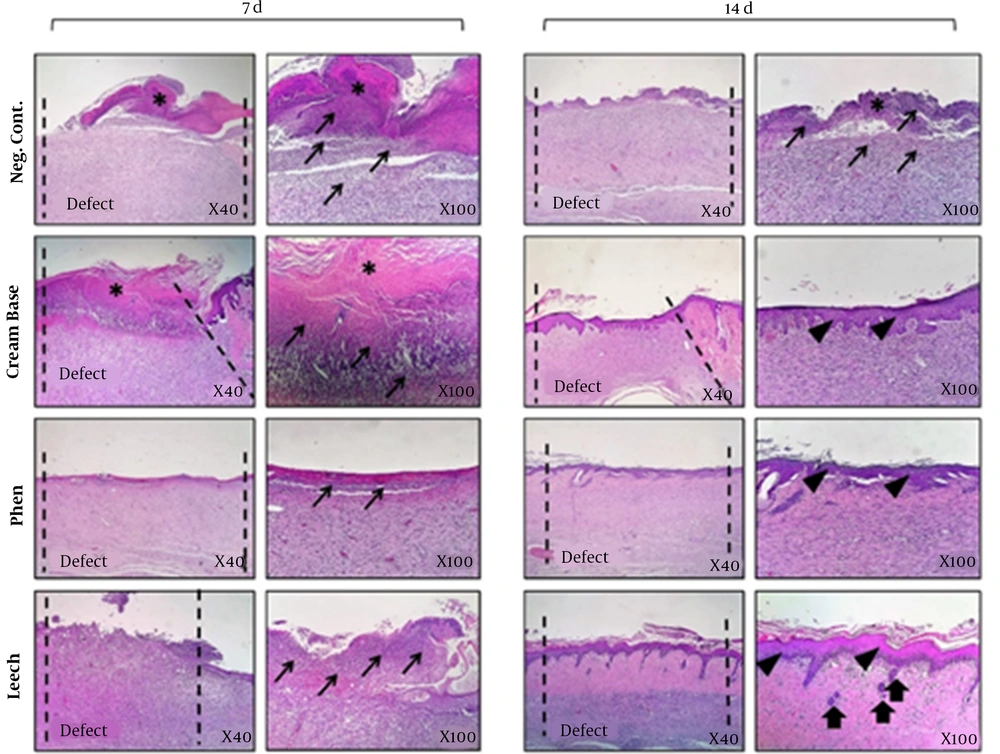
Figure 4 shows H & E staining analysis of rat skin wounds. No healing was observed in the control group, and the histological assessment of the mentioned group during the 7th and 14th of the treatment demonstrated polymorph nuclear corruptive cells (PMNs), penetration and granulation weave development, no observation of skin stratum, and the enclosure of wound was by a hard layer of skin.
Histopathological analysis (H & E stained microscopic sections) of skin wounds in in vivo wound healing model. This study was conducted on rat. Skin samples of 4 treated groups (negative control, cream base, phenytoin and leech) were collected at day 7 and day 14 for histopathological analysis. Epitheliogenesis and angiogenesis of experimental groups were investigated. Leech-treated collection indicated the best outcomes compared to the other collections. Asteroid sign (*): hard skin, thick arrows (↑): Renovation of skin appendages, thin arrows (→): increasing of corruptive cells, Arrowhead (▲): regeneration of epidermal stratum (re-epithelialization).
In addition, the pathological estimation of cream base-treated cluster (cream without any leech) at day 7 was similar to the negative control collection, where a hard scab covered the wound region without epidermal formation and the existence of inflammation in wound region was observed. During day 14, a thin coat of outer skin layers was made, and the amount of inflamed tissue reduced in assessment to the negative control. Regarding phenytoin-treated group, 7-day management demonstrated mild infiltration of corruptive condition into wound area (P < 0.001). However, the number of inflammatory cells decreased compared to cream-based and negative control groups (P < 0.05). The re-epithelialization process was completed in 14th day in this group.
Micrographs of the leech cream-treated indicated the renovation normal appearance of the skin such as adipose glands during the 14th day in post-treatment. This group had more similarity to standard skin at day 14 with the attendance of normal edges, skin appendages, and normal depth of skin stratums. The results confirmed that which leech could exert impressive healing effect on the wounds compared to the control and other treatment groups (Figure 4 and Table 1). Blood vessels were calculated as the average of five regions of each sample under the microscope.
| Group | Epitheliogenesis Score (N = 4) | Blood Vessels/HPF (400×) (7d, N = 4) |
|---|---|---|
| Control | 0,0,0,0 (7 d) | 24.1 ± 3.4 |
| 0,0,1,0 (14 d) | ||
| Cream base | 0,1,0,0 (7 d) | 22.5 ± 3.6 |
| 2,0,1,2 (14 d)* | ||
| Phenytoin | 0,1,1,0 (7 d) | 40.3 ± 4.8** |
| 4,3,4,3 (14 d)*** | ||
| Leech | 0,0,0,1 (7 d) | 45.2 ± 6.1** |
| 4,4,4,3 (14 d)*** |
4.7. Quality (Microbial) Control Test
Based on the results, no growth was observed in microbial colony. Thus, the resent leech product is considered as safe for wound healing purposes (Figure 5).
5. Discussion
Wound healing automatically starts after damage. During the process of healing, it is important to control the cascading reactions of the wound (21). Diabetic foot ulcer is considered as one of the most important wound in the body having a complex treatment (22, 23). There are several treatments for diabetic foot ulcers, among which the use of leeches is considered as one of the recommended methods (24). In general, reducing pain and inflammation is regarded as the first step in wound healing (25), where we can see re-epitelialization and angiogenesis (26). Numerous pharmaceutical products have been developed for achieving these objectives (1, 3, 25, 27-29). Analysis of leech salivary secretion demonstrated more than 90 proteins and peptides with different activities (30). Further, the analgesic effect of leech saliva relies on antistasin with 15 kDa molecular weight which inhibits kallikreins. The inactive forms of kininogens are cleaved to biologically active kinins using proteases such as tissue kallikreins. Kinins mediate stressful situations such as tissue damage or inflammation (31). Eglin C with 8.1 kDa MW is an anti-inflammatory agent for blocking the activity of neutrophil elastase and cathepsin G, which are secreted by activated neutrophils leading to severe tissue destruction (32, 33).
The mast cell Tryptase with 4.3 kDa - 4.8 kDa is considered as a specific inhibitor in leech saliva. Tryptase is a trypsin-like serine proteinase which is available in the extracellular space in tissue samples as abundance and is considered as an indication for mast cell initiation. Tryptase remains active in blood and in the intestine which cannot suppress due to none of the usual inhibitors. Amount of tryptase in serum is correlated with inflammatory situations and anaphylactic responses. The Tryptase inhibitor activity of leech saliva had been proved in this study, which plays an important role in wound healing. The result of the reaction between trypsin and the substrate (Na-benzoyl- L- arginine- 4- nitroanilide hydrochloride) has a specific absorbance in the UV region; in the presence of a Tryptase inhibitor existing in leech saliva which significantly reduces the amount of this absorbance. Inhibitor of C1 complement component prohibits the classical pathway of complement activation, and is served as anti-inflammatory purposes (34). Saratin prevents from binding vWF (von Willebrand) and thrombosis in damage region through binding to exposed collagen I and II, and Hirudin inhibits thrombin leading to blocking formation of fibrin clots with 7.1 kDa MW existing in SGS (11, 13, 35). Our study, anticoagulant activity of leech saliva had been proved by increasing the amount of PTT. In this study, pharmaceutical product was prepared from medicinal leech and the effect of wound healing was evaluated. The result of the present study, especially that of histopathological study, confirms our hypothesis about the wound healing property of the leech saliva proteins. Furthermore, the 5% leech cream was effective enough in wound healing compared to the regular healing product. It seems that hirudin containing anti-coagulation effect can cause a suitable amount of blood flow to the damaged region, where the trypsin inhibitor enzyme inhibits the trypsin; helps protect the wound, and accelerate the healing process.
In addition, two important proteins of the leech saliva products were revealed in this study. Based on the results, other proteins and enzymes in salivary gland secretion of leech such as destabilase with antimicrobial activity, saratin with anti-coagulation activity, and hyaluronidase, calin, glutamyl transpeptidase with special acidities can be helpful in our final product. In this study, we proved that our final product has unique formulation causing skin absorption of proteins and enzymes through the different layers of skin. Finally, our leech cream can be used safely in wound healing, compared to the other wound healing products, and clinical trials are necessary for more accountability.
5.1. Conclusions
The results of anticoagulation and trypsin inhibitory activities of both leech extract and final product indicated the appropriate formulation method. Franz cell in vitro laboratory results for skin uptake indicated the transfer of leech proteins from the membrane. In addition, the results of wound healing confirmed the effectiveness of leech cream formulation.
By considering the results of this study, in the cases where the use of leeches is emphasized such as diabetic foot ulcers, the product can be used instead of direct use of leeches. However, more studies and clinical trials should be conducted in this regard.