1. Background
Burn is a type of injury to the skin or other tissues caused by heat or other reasons that can lead to disability or even death, and the resulting complications can reduce the quality of life. Burns remain a major public health issue all over the world, especially in the developing countries (1). In terms of prevalence, after traffic accidents, falls, and personal violence, burns are the fourth most common type of injury worldwide (2). According to the World Health Organization, about 11 million people suffer burns, which 300,000 of them die (3).
Burns occur when skin cells or other tissues in the body are destroyed by hot liquids, hot objects, flames, chemicals, electricity, radioactivity, and friction with various objects (4). To evaluate the severity of burns clinically, mild to severe injuries are classified in three degrees (5). In first-degree burns, only the outer layer of the skin is damaged, and the skin becomes only hot, red, and painful. Blisters are not seen in this type of burn. In second-degree burns, the outer and middle layers are damaged, which is accompanied by blisters. In third-degree burns, the damage passes through the outer and middle layers of the skin, and the burn may also reach muscle and bone tissue. There is no pain in third-degree burns due to the loss of sensory nerves, but the risk of infection and death in this type of burn injury is high (6, 7).
Since ancient times, the preparation of ointment to reduce pain and accelerate the healing of burn wounds has been considered by physicians. Thus, various substances such as honey, fish oil, aloe vera, and potato have been used to treat burn wounds (8). Advances in wound healing and tissue repair methods have reduced the time of hospitalization and improved the quality of life of burns patients after treatment (9). Today, to treat a burn injury, the skin wound is usually washed, and all skin blisters are removed and cleaned, then the attached tissue is removed, necrotic tissue is shaved off, and standard topical antibiotics such as silver nitrate, mafenide acetate, and silver sulfadiazine are used to treat these wounds (10). Silver sulfadiazine (SSD) belongs to a class of drugs known as sulfa antibiotics. This drug is the most common ointment for dressing second and third-degree burns and is used to prevent and treat burn wound infections (11).
Today, due to the different side effects of many artificial drugs, the use of plant and natural materials has been considered (12). Polyphenols are a group of chemicals found in plants, fruits, and vegetables (12). Phenolic compounds in olive fruit and leaves are among the most important factors with pharmacological properties. Oleuropein (OLE) is one of the most abundant and significant phenolic compounds in olive fruit and leaves, and the bitterness of the olive fruit is due to the presence of this compounds. Oleuropein is a heterocyclic ester of elenolic acid and 4,3-dihydroxyphenyl ethanol (13). Numerous studies have shown that OLE has numerous physiological properties, and the antioxidant, anti-inflammatory, antimicrobial, antiviral, hypolipidemic, and hypoglycemic effects of this compound have been proven in in-vitro and in-vivo studies (13-15). It should be noted that the use of OLE plays a significant role in health (14). The high prevalence of burns and their complications and today’s treatments, which impose staggering costs on families and health care systems, makes it inevitable to find cheaper and more effective therapies.
2. Objectives
A considerable number of studies have shown that OLE has therapeutic effects; therefore, in the present study we evaluated the wound healing activity of OLE in comparison with the reference ointment silver sulfadiazine in a rat model.
3. Methods
3.1. Chemicals
Oleuropein was obtained from Sigma chemicals (St. Louis, Mo, USA). 5,5-dithiobis (2-nitrobenzoic acid (DTNB) and ketamine hydrochloride vials (Rotexmedica, Trittau, Germany) were purchased from Pars biochemistry store, Shiraz, Iran. All other chemicals and solvents used were of analytical grade.
3.2. Preparation of Oleuropein Cream
To prepare the cream, different amounts of OLE (1.25, 2.5, and 5%) were dissolved in eucerin (w/w) at 70°C. The two phases were mixed continuously at the same temperature without vortexing to avoid the entrapment of air. The cream was then cooled down at room temperature while being homogenized at 1500 rpm for 40 min (16).
3.3. Animals
Overall, 72 young male Wistar rats (6 months old, average weight of 200 g - 230 g) were obtained from the animal house of Ilam University of Medical Sciences. They were kept in ventilated cages under standard conditions (temperature of 23 ± 2°C, humidity of 40-50%, with a 12 h light/dark cycle). The rats were fed with standard rat chow and drinking water ad libitum.
3.4. Study Design
3.4.1. Burn Wound Induction
The back hairs of rats were shaved using a shaving machine, and the shaved area was disinfected with betadine solution. Then, the animals were anesthetized by the injection of ketamine hydrochloride (85 mg/kg/ip) and xylazine hydrochloride (6 mg/kg/ip). In the next step to create a second-degree burn, a round metal device made of aluminum with a diameter of approximately 1.5 cm was placed in boiling water at a temperature of 100°C. It was then immediately placed on the shaved area behind the rats for 10 seconds to create a second-degree burn. All the animals were resuscitated immediately with lactate Ringer’s solution (2 mL/100 g body weight/ip). Each rat was then placed in a separate cage and kept in the above-mentioned conditions. Sampling was performed on days 4, 9, and 14, in such a way that the skin tissue was removed around the burn site by injection of 2% lidocaine and using a scalpel number 15, scissors, and forceps (7, 8).
3.4.2. Experimental Groups and Treatments
In the present study, 72 rats were randomly divided into six groups. The animals in all the groups were burned at the beginning of the experiment and were treated as follows:
1- Group 1, Control: The animals in this group remained without any treatment.
2- Group 2, Eucerin Group: Received eucerin twice a day topically for 14 days.
3- SSD Group: The rats in this group received 1% silver sulfadiazine cream twice a day topically for 14 days.
Groups 4 - 6- treatment groups: The wound areas in these groups were covered by 1.25, 2.5, and 5% OLE cream twice a day topically for 14 days.
3.5. Assessment of Wound Healing
On the first, fourth, ninth, and fourteenth days after burn induction, the wounds were photographed using a digital camera (Sony Cybershot DSC-P72). The photographs were then assessed by Image processing software (Digimizer 5.2.2.0, persianGFX.com), and the size of wound area was determined.
3.6. Sample Collection
On days 4, 9, and 14 of the experiment, four rats from each group were anesthetized using a combination of ketamine and xylazine, and the skin of the burnt area was sampled.
One part of the skin sample was placed in 10% formalin and sent to the laboratory for histopathological examination, and the other part of the skin tissue was homogenized with a phosphate buffered saline (PBS) and kept at -20°C for the evaluation of glutathione (GSH), malondialdehyde (MDA), and hydroxyproline (HP) levels and inflammatory factors.
3.7. Histopathological Examination
For histological studies, the samples were fixed in formalin solution, dehydrated with a sequence of ethanol solutions, embedded in paraffin, cut into 5-µm sections, and colored with hematoxylin and eosin dye (H & E stain). The slides were observed under OLAMPUS light microscope to be examined for the presence of re-epithelialization, angiogenesis, collagen synthesis, inflammatory changes, polymorphonuclear cells, and fibroblasts (17).
3.8. Estimation of Tissue Biochemical Parameters
The skin tissues were divided into small pieces and homogenized in ice-cold PBS (50 mM, pH = 7.4) at a concentration of 10% (w/v). Then, the tissue homogenate was centrifuged at 3000 rpm for 15 minutes at 4°C, and the supernatant was separated for the measurement of biochemical parameters. Protein concentration was also measured using Bradford instructions (18). The supernatant was used for the evaluation of HP content, GSH, MDA, transforming growth factor beta (TGF-β), and Interleukin 6 (IL-6) by standard methods.
3.8.1. Measurement of MDA
The amount of MDA in the skin tissue was measured using an MDA kit made by Zellbio Company. Briefly, MDA reacts with thiobarbituric acid (TBA) at high temperatures to produce a pink color, which is measured by the colorimetric method at a wavelength of 540-530 nm. The amount of MDA was expressed as n mol/mg protein.
3.8.2. Determination of GSH Content
Glutathione content of the skin tissues was measured by the reaction of reduced glutathione with Elman reagent (DTNB) (19). In summary, 1 mL of the skin tissue homogenate was added to 2 ml of Elman reagent and mixed. Then, the absorbance of the mixture was read by a spectrophotometer at 412 nm. Reduced glutathione was used to draw the calibration curve. Glutathione content was expressed as µmols/mg protein.
3.8.3. Estimation of HP Content of the Ulcer
The amount of HP in skin tissue was measured according to the Woessner method (20). For this purpose, 9 mL of HCl (6 N) was added to 1 mL of homogeneous tissue sample, and the samples were placed in heat-resistant tubes at 120°C for 16 hours. Hydrolyzed samples were neutralized using NaOH (2.5 N) and two drops of methyl red. The oxidation step was then started by adding 1 ml of chloramine-T solution (1.41 g of chloramine-T in 20 mL of water, 30 mL of isopropyl alcohol, and 50 mL of citrate/acetate buffer) to 2 mL of the sample. After 20 minutes, 1 mL of Ehrlich reagent (20% Para Dimethylamino benzaldehyde solution in perchloric acid and isopropyl alcohol) was added, and the samples were placed in water bath at 60°C for 20 minutes. The absorption rate was then calculated by a spectrophotometer at 577 nm. Concentrations of 0 - 10 µg/mL of HP solution were used to prepare the standard curve, and the amount of HP was reported as mg/g tissue.
3.8.4. Measurement of TGF-β and IL-6
Abcam’s enzyme-linked immunosorbent assay (ELISA) kit was used to measure TGF-β and IL-6 in the rat skin homogenate tissue. Rat IL-6 ELISA kit is a single-wash sandwich ELISA designed for the quantitative measurement of IL-6 protein in the range of 125 pg/g tissue - 8000 pg/gr tissue with a sensitivity of 43 pg/mL. Abcam’s TGF-β rat ELISA kit quantitatively measured rat TGF-β in the range of 31.3 - 2000 ng/g tissue with a sensitivity of 8 ng/g tissue. All the measurements were done according to the company instruction.
3.9. Ethics Statement
This study was approved by Ilam University of Medical Sciences with the institution’s research ethics board approval number (code: IR.MEDILAM.REC.1399.309). This research was conducted in compliance with the research ethics standards.
3.10. Statistical Analysis
Graph Pad Prism software (version 5) was used for statistical analysis. For each group, the mean level of the variable was calculated as mean ± SEM. Multiple comparisons between different groups was performed using one-way ANOVA followed by Tukey’s post-hoc test. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. The Effects of OLE on the Burn Wound Size
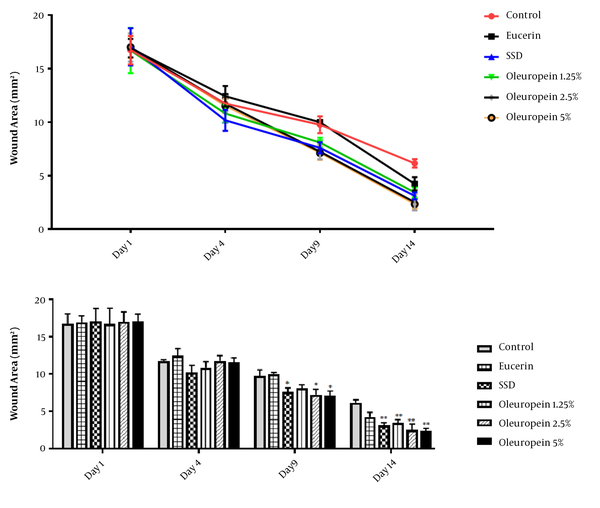
On the first day after burn induction, the size of the wound area was similar in all groups, and no significant difference was observed between different groups (P > 0.05). Comparison of wound size in different time periods showed a continuous reduction in wound size over time.
On the fourth day, wound size was decreased in all the groups, but there was no significant difference between different groups (P > 0.05).
Also, on the 9th and 14th days, there was a significant difference in wound contraction between different groups, and the groups receiving SSD and different doses of OLE cream showed a significant difference as compared to the control group (P < 0.05). Moreover, there was no significant difference between groups 4-6 on the 9th and 14th days (P > 0.05). The results of wound size measurement are shown in Figure 1.
4.2. The Effects of OLE on HP Content
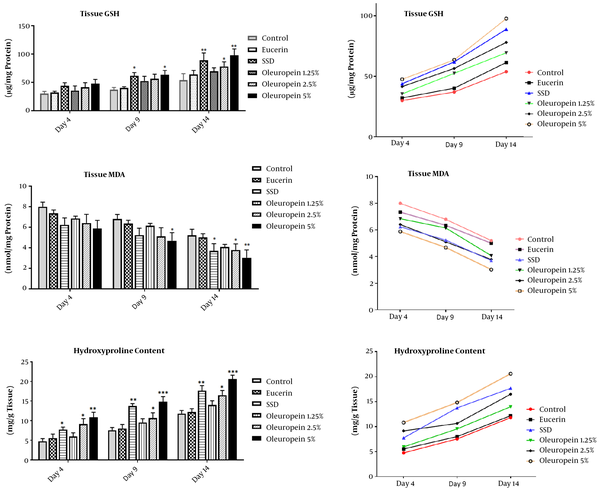
The measurement of HP content in the wound area showed that this compound increased during the test period. At all the three time points (days 4th, 9th, and 14th), the groups receiving SSD and 2.5 and 5% of OLE showed a significant increase in this regard compared to the control group (Figure 2).
The treatment effects of oleuropein on oxidative stress biomarkers (MDA and GSH) and HP content in different groups on the fourth, ninth, and fourteenth days of the experiment. Each value represents means ± SEM. *, Significantly different from the control group; *, P < 0.05; **, P < 0.01; ***, P < 0.001
4.3. The Effects of OLE on Burn-Induced Oxidative Stress
Malondialdehyde and GSH were measured in the burn area tissue as indicators of oxidative stress. An analysis of MDA level revealed that the amount of this compound in wounds was markedly decreased on days 4, 9, and 14 after the burn induction. On the 4th and 9th days, there were no significant differences between the groups, but on the 14th day, SSD and OLE (2.5 and 5%) groups showed a significant reduction compared to the control group (Figure 2).
The examination of GSH content in different groups exhibited that GSH increased over time in wound tissue. On the 4th day, there was no significant difference between the groups. On the 9th and 14th days, in the SSD and high dose of OLE (5%) groups, GSH content markedly increased compared to the control and eucerin groups (Figure 2).
4.4. Inflammatory Factors
To investigate inflammatory tissue factors, IL-6 and TGF-β were measured in the rat skin homogenates. The results of IL-6 measurement showed that on the 4th day, no significant difference was observed between the experimental groups. On the 9th and14th days, the levels of IL-6 in the treatment groups (SSD and 1.25, 2.5 and 5% of OLE) were less than the control group; however, only group 6, which received 5% OLE cream showed a significant reduction compared to the control group (P < 0.05 on the 9th day and P < 0.01 on the14th day). It should be noted that the amount of IL-6 showed a significant decrease over time in the experimental groups (P < 0.05; Table 1). Regarding TGF-β level, there was no significant difference between different groups on the 4th day. On the 9th day, the level of TGF-β in group 6 which received 5% of OLE cream was significantly lower than the control group (P < 0.05). On the 14th day, the treatment groups that received SSD and 2.5 and 5% of OLE showed a significant reduction compared to the control group (P < 0.05, P < 0.01, and P < 0.001, respectively; Table 1).
| Control | Eucerin | SSD | Oleuropein 1.25% | Oleuropein 2.5% | Oleuropein 5% | |
|---|---|---|---|---|---|---|
| Il-6, pg/g tissue | ||||||
| Day 4 | 1024 ± 125 | 974 ± 75 | 909 ± 78 | 974 ± 92 | 899 ± 88 | 802 ± 73 |
| Day 9 | 918 ± 79 | 898 ± 62 | 669 ± 57 | 776 ± 84 | 667 ± 67 | 619 ± 57* |
| Day 14 | 732 ± 60 | 651 ± 45 | 489 ± 49 | 601 ± 58 | 535 ± 78 | 391 ± 48** |
| TGF-b, ng/g tissue | ||||||
| Day 4 | 401 ± 39 | 356 ± 38 | 346 ± 49 | 366 ± 35 | 316 ± 49 | 296 ± 38 |
| Day 9 | 435 ± 44 | 426 ± 42 | 306 ± 39 | 376 ± 30 | 286 ± 29 | 249 ± 27* |
| Day 14 | 323 ± 40 | 282 ± 30 | 184 ± 28* | 251 ± 19 | 162 ± 28** | 149 ± 18** |
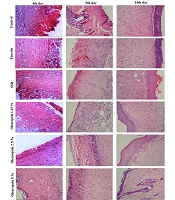
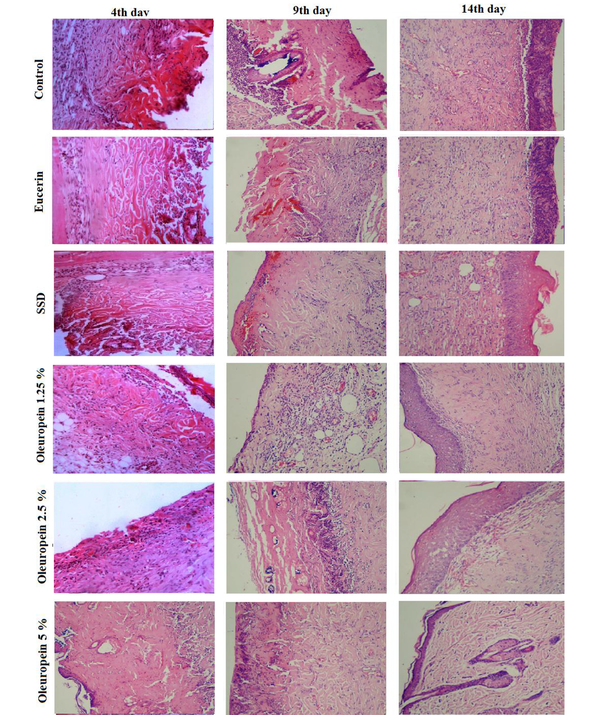
4.5. Histopathological Findings
Histopathological examination with light microscopy showed that on the fourth day after injury, severe inflammation, crust formation, and angiogenesis were seen in all the experimental groups. On the ninth day, in the eucerin and control groups severe inflammation and mild angiogenesis were noted. In the SSD and OLE cream groups, connective tissue matrix dispersion, irregular granulation, and inflammation were lower than in the control group. Also, the thickness of the epithelium and dermis in the groups receiving SSD and OLE with the concentrations of 2.5% and 5% were higher compared to the control group. On the 14th day, collagen deposition was observed in all the experimental groups. Epidermal formation in the SSD and OLE treatment groups showed a higher quality than the control group. Also, more regular arrangement and lower inflammation were observed in the treatment groups (Figure 3).
5. Discussion
The main purpose of burn management is healing wounds and accelerating the epithelialization process (11, 21). Wound healing is a collaborative process and consists of three stages, including inflammation, cell growth, and cell maturation (22). Burn wounds have a low healing capacity; thus, they cause critical clinical problems such as infection, necrosis, and gangrene. Therefore, many efforts have been made to accelerate the healing of burn wounds (7). Different studies indicated that natural antioxidant compounds have therapeutic effects in wound healing (23, 24). Oleuropein, the main phenolic component of olive, has several health effects such as antioxidant and anti-inflammatory activities (25, 26). Therefore, this finding motivated us to investigate the potential effects of OLE on the repair of superficial skin burn in a rat model.
The average wound area is used as one of the indicators to assess the process of burn wound healing. The evaluation of wound size showed that SSD and OLE-treated groups had a significantly accelerated wound healing and wound size reduction compared to the control group, which indicates the effect of these compounds on wound healing. The findings of this study also showed that high doses of OLE (5%) had the greatest effect on accelerating wound healing. This result was also supported by histological findings, which indicated the elevation of collagen deposition, angiogenesis, and minimal inflammation in the OLE-treated groups.
Immediately after wound induction, the damaged arteries constrict. After a few minutes, the blood vessels dilate to increase blood flow to the site of injury for reconstruction, and then the inflammatory phase begins (8). The main features of the inflammatory phase are the presence of inflammatory cells (i.e., neutrophils, mast cells, and basophils) and the production and secretion of inflammatory factors, including a variety of cytokines, prostaglandins, and interleukins by inflammatory cells at the wound site. Bacterial infections at the wound site cause more neutrophils to migrate to the wound site, which can lead to tissue damage and prolong the inflammatory phase, thus increasing the healing time of damaged tissue. This causes severe pain in the early stages and the production of excessive scar tissue at the wound site.
Our findings showed that the rates of edema and tissue inflammation were much lower in the groups treated with SSD and high doses of OLE (2.5 and 5% of OLE) compared to the eucerin and control groups. Also, wound treatment with OLE cream reduced inflammatory factors TGF-β and IL-6 at all the three time points.
Other researchers have reported the anti-inflammatory properties of OLE in various diseases. For example, in a study conducted by Khalatbary and Zarrinjoei (27), the anti-Inflammatory effect of OLE in spinal cord trauma was investigated. Their findings indicated that OLE modulates inflammatory reactions following spinal cord injury and attenuates TNF-α, IL-1ß, nitrotyrosine, iNOS, and COX-2 activity in this injury (27).
In another study by Qabaha et al. (28), the anti-inflammatory effect of OLE on the polymorphonuclear cells of whole blood was evaluated. In this study, tumor necrosis factor α (TNFα) level was measured after lipopolysaccharide stimulation. Their findings showed that OLE treatment led to the downregulation of TNFα secretion in PMNCs culture, indicating that this compound has an anti-inflammatory effect (28).
Barbaro et al. (25) believes that OLE exerts its anti-inflammatory effects through inhibiting the biosynthesis of pro-inflammatory cytokines or modulating inflammatory parameters. Following the wound healing process, with the reduction of edema and tissue inflammation, the second stage of the healing process begins with vascular regeneration and migration of fibroblasts and fibrocytes to the wound site, resulting in the production and secretion of higher amounts of collagen, elastin, and proteoglycans at the wound site (29). Collagen is the major protein of the extracellular matrix and leads to the increase of wound contraction. Accordingly, assessing the amount of collagen deposition in the wound area can be an indicator of wound healing speed (29). Hydroxyproline is a major component of the protein collagen, and it has been widely measured as an indicator of collagen synthesis in the wound healing process (30). In the present study, it was observed that in groups 5 and 6, which received high doses of OLE, the rate of inflammation decreased more than the control group over time. Also, the amount of tissue HP in these groups was higher than the control group, which indicates the potential of this substance in reducing inflammation and stimulating collagen production.
Free radicals are formed in burn wounds and lead to tissue damage, which can be controlled with antioxidant compounds (31). In the present study, the amounts of GSH and MDA were also measured as indicators of oxidative stress in the tissue. The findings showed that OLE ameliorated oxidative stress in the treatment groups and led to the reduction of MDA and increased GSH. These findings are consistent with those of studies by other researchers who evaluated the antioxidant properties of OLE. For example, in a study by Jemai et al. (32), the lipid-lowering and the antioxidative activities of OLE in a rat model were investigated. Their findings showed that OLE was able to reduce serum lipid levels and lipid peroxidation and increase the activity of antioxidant enzymes (32). In another study, Al-Azzawie and Alhamdani (33) demonstrated the antioxidant effect of OLE in alloxan-diabetic rabbits. They suggested that the administration of oleuropein prevented diabetic complications associated with oxidative stress (33).
5.1. Conclusions
Our findings showed that the beneficial effects of OLE in the wounds healing are comparable with SSD. Moreover, these findings suggested that the promotion of burn wound healing by this compound may be attributed to its antioxidant and anti-inflammatory properties and stimulation of collagen synthesis. Thus, this natural compound can be considered as an alternative treatment for burns healing.