1. Background
Acne is the most common chronic skin disease affecting young adults and adolescents worldwide. The characteristics of this disease include chronic inflammation of sebaceous glands in the face, neck, and upper parts of the body leading to open and closed comedones (blackheads and whiteheads), papules, pustules, nodules, and cysts (1-3). Several factors affect the development and progression of acne, including genetics, hormonal status, bacteria, especially Propionibacterium, and stress (4, 5). Based on the type of inflammatory lesions, the severity of acne is graded as mild, moderate, and severe (6). Besides, based on the number of lesions, it is classified into four groups: mild, moderate, severe, and very severe.
Typically, topical medicines such as tretinoin and antibiotics and systemic drugs, including oral antibiotics and isotretinoin, are commonly used to treat acne (7). High propionic resistance of the acne bacterium to these antibiotics, as well as high cost, side effects, and complications of treatment failure, have been reported (8). Due to the efficacy and safety of plants, they can have significant applications in treating acne and other skin infections. Licorice (Glycyrrhiza glabra L.), an herb belonging to the Fabaceae family, contains many compounds such as various sugars, flavonoids, sterols, amino acids, gum, starch, oily essence, and saponins used in traditional Asian and European medicines for the treatment of gastritis, respiratory infections, peptic ulcers, and so on (9-14). Licorice has significant antibacterial activity against Propionibacterium acnes and prevents acne formation (15). Pomegranate (Punica granatum L.) is a member of the Lythraceae family whose products are now being used to treat AIDS (16), produce cosmetic products (17), and protect against cardiovascular diseases (18). Different parts of pomegranate, such as its fruit peel and leaves, contain various phenolic substances with antimicrobial properties (19). Studies indicate that pomegranate has a powerful effect on acne and can be used in anti-acne compounds due to its antimicrobial, anti-lipase, and anti-inflammatory effects (16).
Aloe vera L. is a plant of the Liliaceae family with application in cosmetic products (20). So far, 75 compounds have been found in Aloe vera, including 20 types of minerals, 20 amino acids, vitamins, polysaccharide glucomannan compounds, carboxypeptidases, magnesium, zinc, calcium, glucose, cholesterol, salicylic acid, prostaglandins, vitamins A, C, and E, lignins, saponins, herbal sterols, and amino acids (21, 22). Aloe vera is a medicinal herb applicable to various diseases and skin lesions as a topical remedy. The effectiveness of topical products containing Aloe vera in reducing acne lesions was significantly better than that of placebo (23). Zataria (Zataria multiflora), a plant belonging to the Lamiaceae family, has a long history of use in Iranian traditional medicine. It has been used to treat gastrointestinal disorders, local ulcers, and respiratory and catarrhal disorders due to its decongestant and sputum effects (24). The significant components of Zataria essence are carvacrol, thymol, linalool, and p-cymene. Thymol and carvacrol are the most important active components of Zataria essential oil (25).
2. Objectives
Considering the mechanisms associated with acne formation and studies confirming antimicrobial, anti-inflammatory, antioxidant, and phytoestrogenic effects of the plants, in this study, we aimed to prepare a formulation containing these herbs to treat acne and compare it with a commonly used anti-acne preparation, ie, clindamycin gel.
3. Methods
3.1. Medicines Preparation
The herbal cream was prepared at the Faculty of Pharmacy, Jundishapur University of Medical Sciences, Ahvaz, Iran. To this end, Aloe vera leaves, pomegranate peel, licorice root, and Zataria leaves were provided from reputable sources. After confirmation of the herbs at the Medicinal Plants Research Center, the extracts were prepared by a maceration process. The plants were powdered, soaked in adequate amounts of ethanol 70° in glass flasks, and stored at room temperature for 48 hours to extract. Finally, the extracts were condensed by a rotary evaporator at 45ºC and dried using a freeze dryer for 72 h. To prepare the Aloe vera extract, the leaves were cut and washed with water. The thick epidermis was removed, and the inner gel of the leaves was separated with a spoon and finally homogenized in a mixer (26).
A suitable formulation containing 5% pomegranate peel extract, 4% licorice root extract, 8% Aloe vera extract, and 2% Zataria extract was prepared from total extracts. The extract concentrations were selected based on previous studies with some modifications to obtain the best formulation. The components of the oil phase, including soft and liquid paraffin (20%), stearic acid (5%), cetyl alcohol (1%), beeswax (15%), and triethanolamine (1%), were melted at 60ºC and mixed. The aqueous phase components, including borax (1%) and glycerin (5%), were dissolved in deionized double-distilled water. Both phases were mixed at the same temperature. Continuous and quick stirring was applied until the cream coagulated and became utterly homogenous. After preparation of the cream base, the extracts were added at desired concentrations, mixed gently, and homogenized.
The 1% clindamycin gel from the Iranian pharmaceutical market was used for the control group. The gel was removed from the tubes and poured into the tubes similar to the herbal cream tubes; the tubes were then coded. The unique codes provided by the software were used on drug boxes. None of the people involved in the study were aware of the assigned groups, except the statistical consultant.
3.2. Patient Selection
This research is part of a randomized, double-blind, two-arm parallel groups, controlled study to investigate the clinical efficacy of the herbal cream in subjects with mild-to-moderate acne. Patients were randomly assigned at a 1:1 ratio to receive the herbal cream or 1% clindamycin gel, with a computer-generated randomization sequence (blocks of 2 - 4). The tubes were identical in appearance, packaging, and labeling to ensure masking between the groups. Physicians and patients were blind to the intervention. It included 66 patients with mild-to-moderate acne. Mild acne was defined as a non-inflammatory lesion with the number of papules and pustules less than 10 without any nodes or cysts. Moderate acne was described as a non-inflammatory lesion with the number of papules and pustules less than 20 without any nodes or cysts.
Patients referred to Al-Zahra Hospital of Isfahan, Iran, meeting the inclusion criteria were randomly divided into two groups. The inclusion criteria included patients with mild-to-moderate acne, not taking topical medications in the past one month or oral anti-acne medications/oral contraceptive pills (OCPs) in the past two months, not being lactating, and having no allergy to clindamycin. The exclusion criteria included lactation, other skin diseases, and an allergy to the herbal cream.
3.3. Clinical Trial
The present study was a double-blind, randomized clinical trial with a code of IRCT20181026041466N1 obtained from the Iranian registry of clinical trials (IRCT). All patients who met inclusion criteria in Al-Zahra Hospital in Isfahan were randomly assigned into two groups. The patients filled in the informed consent forms and randomly received a herbal medicine or gel packet. The instruction for use was explained to the patients. For allergy testing, each group of patients was asked to use their medication on their arms; if no allergic reaction occurred, the remedy would be applied to the face for acne. The treatment period was two months, and participants were asked to softly wash their faces with confirmed cleansers and warm water, then gently patting their faces dry. They applied the herbal cream or clindamycin gel three times daily on the acne-affected area for eight weeks. Their photographs were taken before and after receiving the drug, and the total acne lesion count (TLC) and acne severity index (ASI) were measured. Patients were examined every four weeks for adverse reactions such as erythema, irritation, itching, light sensitivity, drug intolerance, and other complications by taking the history and clinical examination.
3.4. Measurement Indices
The ASI was used to determine the severity of acne, calculated for each patient as follows:
ASI = (number of pustules × 2) + number of papules + (number of comedones ÷ 4) (27)
The reduced ASI of less than 30% was considered a weak response, 30% to 60% moderate, and over 60% excellent.
The TLC showed the total number of acne lesions, calculated as follows:
TLC = Number of pustules + number of papules + number of comedones + number of nodules (27)
3.5. Statistical Tests
Descriptive statistics such as frequency distribution, mean, and standard deviation were used. The Shapiro-Wilk test was used to investigate the data normality. A two-sample independent t test and the chi-square test were used to compare the homogeneity of demographic characteristics and the severity of the main variables between groups at baseline. The mean changes in the measured variables were assessed using statistical one-way repeated measures ANOVA to evaluate the intra- and inter-group effects. The effects of two independent factors, namely treatment group (at two levels of intervention and control groups) and time (at three levels of assessment time before intervention, one month after intervention, and two months after intervention), were investigated. We used SPSS software ver. 20 to analyze the data. A P value equal to or less than 0.05 and 95% confidence intervals were employed to determine the significance level.
4. Results
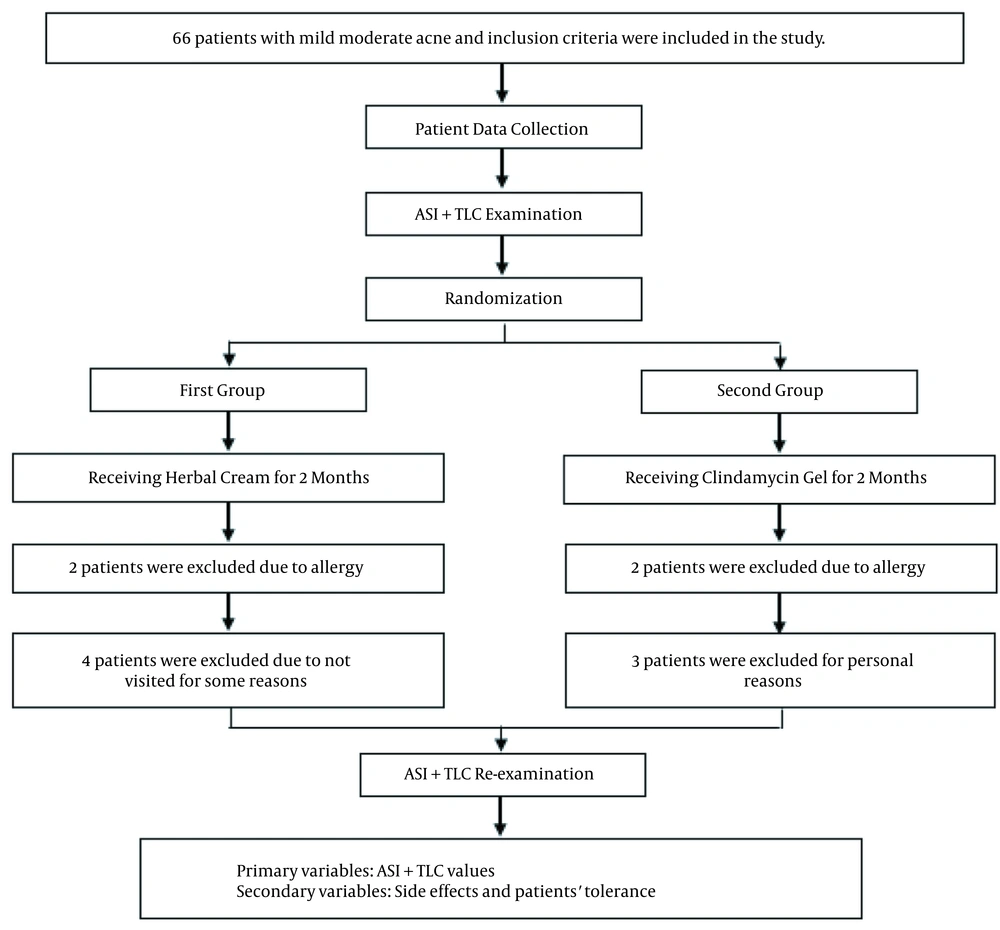
Sixty patients with mild to moderate acne were studied in this study, including 42 females and 18 males. Two patients in the herbal cream group and two in the clindamycin gel group were excluded due to allergy reactions. One patient was also excluded due to the improper use of medicines and personal issues. Finally, 55 participants completed the study. Table 1 represents the patients' demographic characteristics, and Figure 1 shows the flowchart of the study.
| Values | |
|---|---|
| Gender (n = 60) | |
| Male | 18 |
| Female | 42 |
| Age (n = 60) | |
| Under 20 | 20 |
| 20 - 30 | 32 |
| Over 30 | 8 |
Table 2 represents the results of clindamycin gel-treated and herbal cream-treated groups based on the type and number of lesions assessed by the physician.
| Scale | Before Treatment | After 4 Weeks | After 8 Weeks | P-Value |
|---|---|---|---|---|
| Herbal cream | ||||
| ASI | 13.25 ± 6.6 | 7.02 ± 4.26 | 6.25 ± 4.1 | 0.00029* |
| TLC | 19 ± 9.4 | 10.70 ± 7.02 | 9.40 ± 6.88 | 0.0003* |
| Clindamycin gel | ||||
| ASI | 10.06 ± 5.8 | 6.64 ± 4.45 | 5.55 ± 4.1 | 0.000296* |
| TLC | 15.20 ± 6.52 | 11.85 ± 6.09 | 10.18 ± 5.9 | 0.0004* |
a Values are expressed as mean ± SD.
Based on statistical analysis, both drugs showed significant effects (P < 0.005) on the ASI decrease after two months. The mean ASI was decreased as 52.83% and 44.83% after two months in the herbal cream and clindamycin groups, respectively. Even though the herbal cream led to higher recovery than clindamycin, there was no significant difference in the ASI between the two groups (P = 0.179). Meanwhile, the rate of this index was not significantly different between the two groups before the treatment.
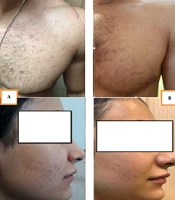
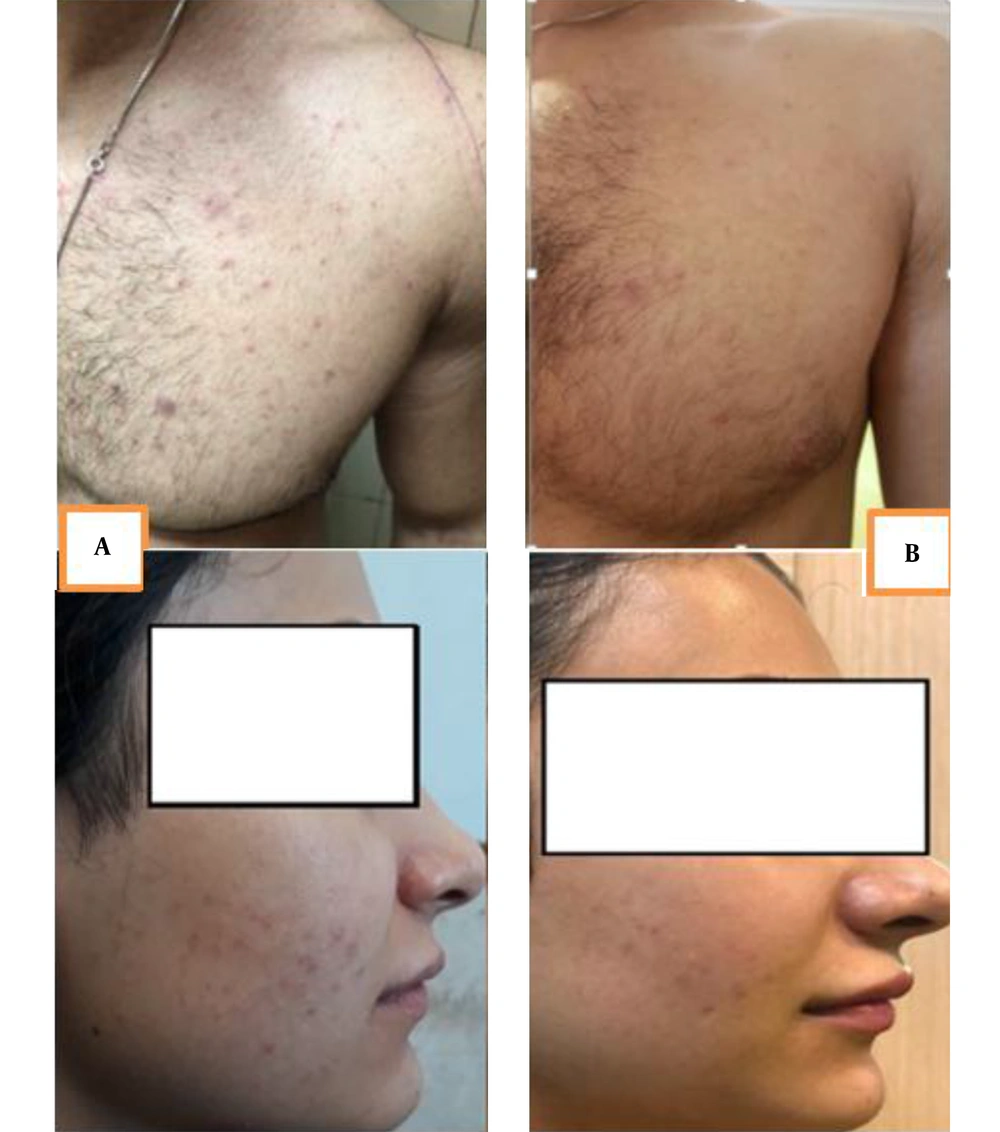
The results indicated that the effects of both drugs were significant on the TLC index after two months (P < 0.005). Although the herbal cream was more effective than clindamycin in decreasing TLC (51.55% and 33.03%, respectively), no significant difference was found between the two groups. There was also no significant difference between the two groups before treatment. Figure 2 shows the photography of two subjects before and after treatment by the herbal cream.
5. Discussion
Acne is the most common chronic skin disease affecting 85% of adolescents and young adults worldwide. However, acne can continue in adulthood, especially in women after the age of 25. This disease affects millions of adolescents every day (1).
Acne is caused by several factors, including the propionic activity of acne bacterium, increased sebum production, androgenic stimulation, follicular duct hyperkeratosis, inflammatory response (lymphocytic, macrophagic, and neutrophilic), and cytokine activity (4, 5). Topical medicaments such as tretinoin and antibiotics and systemic productions such as oral antibiotics and isotretinoin are commonly used to treat acne. The reported microbial resistance in acne is 13% for erythromycin, 10% for clindamycin, and 1.7% for co-trimoxazole. The report indicates high resistance of Propionibacterium acnes to these antibiotics, associated with side effects of antibiotics and complications of treatment failure, which can increase the costs (7, 10).
This study planned to create and clinically use an herbal formulation containing several herbs with antimicrobial, anti-inflammatory, antioxidant, and phytoestrogenic effects for acne treatment. Glycyrrhiza glabra L. (licorice) is a perennial herb from the Fabaceae family. It contains several compounds such as various sugars (up to 18%), flavonoids, sterols, amino acids, gum, starch, essence, and saponins (11, 13). This plant has significant antibacterial activity against the P. acnes bacterium. As reported, the inhibitory effect of licorice on P. acnes growth is not associated with bacterial resistance in vitro. It can also prevent acne and wound lesions (15). Pomegranate, Punica granatum L., is a member of the Punicaceae family and a natural source of phenolic compounds and antioxidants such as tannins, polyphenols, flavonoids, and vitamin C. The fruit peel and leaves contain various phenolic substances responsible for the antimicrobial activity of the extract. Several studies have found that the antimicrobial activity of pomegranate extract may be due to the presence of some compounds with antimicrobial activity (19-28). The pomegranate peel extract significantly reduces P. acnes-induced edema in rats' ears. The extract inhibits the growth of bacteria and lipase activity. Studies have found that the extract has a powerful effect on acne, which can be used in anti-acne preparations. For example, Lee et al. showed that pomegranate peel extract could significantly affect inflammation induced by acne vulgaris (16).
Aloe vera is a plant from the Liliaceae family used as a topical remedy for various diseases and skin lesions (23). It contains salicylic acid, and magnesium lactate that primarily acts as an anti-inflammatory and analgesic agent via inhibiting the prostaglandin production and secondary inhibits the histidine decarboxylase and conversion to histamine in mast cells. Hence, it has an anti-itching effect (29). Aloe vera is also effective on various bacteria, including Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumonia (30). The Aloe vera plant can be topically applied to control and treat acne. A study indicated that a topical product of Aloe vera was significantly better than a placebo in reducing acne-induced lesions (24). A clinical study by Hajheydari et al. showed that a combination of tretinoin and Aloe vera gel could be significantly more effective than tretinoin alone on acne vulgaris with reduced microbial tolerance (31).
Zataria multiflora contains fatty acids, oleanolic acid, β-sitosterol, Betolin, carvacrol, thymol, linalool, and p-cymene in aerial parts. It has anti-inflammatory, antioxidant, and antimicrobial effects (25). Flavonoids have anti-phosphodiesterase activity and thus can increase the levels of circular intracellular nucleotides. Recent studies indicate that both cAMP and cGMP can reduce oxidative stress in many biological systems and diseases (32).
No drug comprehensively improves acne or prevents its recurrence. Furthermore, some pathogens are resistant to medications (33) in which oral and topical antibiotics play essential roles, so drug resistance is a significant problem resulting from the widespread use of these antibiotics in acne treatment. Plenty of plants with anti-inflammatory and antioxidant properties are used to treat acne alone or in combination with other plants like green tea, fenugreek, turmeric, and coffee (34). In this study, four plants with different properties were used to prepare a formulation to cover all aspects of acne treatment, aiming to investigate whether the combination of these plants can be effective in treating acne.
According to the results, although the decreased TLC and ASI after two months were significantly different in both treatment groups, the difference between the two groups was not statistically significant. The present study compared the herbal cream to 1% clindamycin gel as a positive control. Clindamycin was chosen, especially for its availability and tolerability. Clindamycin is the commonly prescribed topical antibiotic for acne vulgaris with anti-inflammatory properties. Herizchi Qadim et al. evaluated the impact of 1% clindamycin gel on mild to moderate acne and indicated a significant reduction in the indices after eight weeks. The ASI and TLC decreased from 7.17 ± 2.46 to 2.11 ± 1.22 and 15.56 ± 3.82 to 5.03 ± 2.42, respectively (35). Furthermore, Shahmoradi et al. examined the anti-acne effects of clindamycin hydrochloride gel and nicotinamide gel. The results of this clinical study indicated that clindamycin hydrochloride gel and nicotinamide gel had favorable anti-acne outcomes (36). Based on the results of this study and considering the increasing global acceptance of the use of herbal medicines, it can be concluded that due to the low side effects, less use of chemical antibiotics, and greater patient satisfaction, this herbal cream can be used in the treatment of acne lesions.