1. Background
Hyperpigmentation disorders of the skin are a group of conditions that cause darkening of the skin, with melasma and post-inflammatory hyperpigmentation (PIH) being the most common types. The exact etiologies of melasma and PIH are unknown, but external factors such as prolonged sun exposure, women’s hormonal fluctuations, and drug adverse effects have been indicated (1, 2). These disorders are of great psychological importance since they can negatively impact the quality of life and emotional well-being of an individual. This importance has been demonstrated in various studies (2).
First-line treatment of these disorders is usually carried out topically with hydroquinone, which is commonly used concomitantly with retinoids and sometimes topical corticosteroids. Hydroquinone interferes with melanin synthesis, while retinoids increase keratinocyte turnover, and corticosteroids are used to reduce the inflammation caused by the retinoids and hydroquinone. Additionally, corticosteroids have a non-selective inhibitory effect on melanogenesis. However, these agents and other commonly utilized medicines frequently cause skin irritation. In addition, hydroquinone may cause contact dermatitis, and long-term usage of this drug is associated with conjunctival melanosis, nail discoloration, and a rare, yet serious adverse effect called exogenous ochronosis. On the other hand, chronic topical corticosteroid therapy may cause acne, epidermal atrophy, and striae (3, 4). Accordingly, there has been an ongoing research to find alternatives, especially herbal ones, for hydroquinone and other compounds. Examples of such alternatives include soy extract, licorice extract, arbutin, green tea, and Aloe vera; some of these compounds have been used as empiric therapy in traditional medicine (5).
Morus alba L. (common name: white mulberry) is a Chinese tree utilized for fodder and silk production. Traditionally, different parts of the plant, such as leaves, fruits, and root bark, have been used for treating conditions such as common cold, pain, high blood pressure, diabetes, urinary incontinence, and constipation (6). It has a wide range of active constituents, including flavonoids, anthocyanins, stilbenoids, terpenoids, alkaloids, phenolic acids, and coumarins. Some of the many pharmacological effects of the plant that have been demonstrated in various studies are antioxidant, anti-inflammatory, antimicrobial, antidiabetic, and antihyperlipidemic effects.
Skin lightening properties have been demonstrated by crude extracts or isolated phenolic compounds from root barks, twigs, and leaves of the plant, both in vitro and in vivo (7). Some of the isolated compounds include oxyresveratrol and its mono- and di-glycosylated (mulberroside A) forms, moracin M and its glycosylated form mulberroside F, kaempferol, quercetin, and its glycosylated form isoquercetin, rutin, maclurin, resveratrol, and morin (8-12). So far, no study has attempted to develop a suitable formulation from the leaves of white mulberry, perform its stability studies, assess microbial growth, and assay total phenolic compounds as the active ingredients.
2. Objectives
The aim of this study was to develop a topical formulation obtained from crude (70% ethanolic) extract of white mulberry leaves and evaluate its stability, physicochemical properties, microbial growth, and preservative effectiveness under accelerated conditions.
3. Methods
3.1. Materials
White mulberry leaves were gathered in May 2017 from the green space of Tehran, Iran by the main author and later authorized by a certified botanist at the herbarium of the Faculty of Pharmacy of Tehran University of Medical Sciences (voucher number: TEH-6599).
Ninety-six percent ethyl alcohol and titanium dioxide were bought from Zakariajahrom (Iran) and Sepidaj (Iran), respectively. Folin-Ciocalteu’s reagent, cetearyl alcohol, cetyl palmitate, isopropyl alcohol, methylparaben, propylparaben, isopropyl myristate, benzyl alcohol, ethylenediaminetetraacetic acid (EDTA), Span 60, Tween 80, gallic acid, sodium bicarbonate, and hexane were purchased from Merck (Germany). Sepigel 305 was obtained from Seppic (USA). Deionized water was prepared freshly as required.
3.2. Preparation of the Extract
First, the leaves were dried under room temperature at 25°C in the shade and then crushed slightly by hand. To extract the total phenolic contents, 70% ethanol was used. Seventy percent ethanol was chosen over the 96% ethanol as the solver after determining the extraction yield for 1 g of dried leaves by measuring the dried extract weight. Next, repeated maceration was performed for 1 kg of dried leaves using 70% ethanol. The process was carried out three times, each for 72 hours. Finally, the extract was concentrated using rotary evaporator Heidolph (Model: Hei-VAP Value Hand Lift; Germany). The extract was stored in a closed glass container in refrigerator at 7 ± 1°C and was used as needed.
3.3. Quantification of the Phenolic Content of the Extract
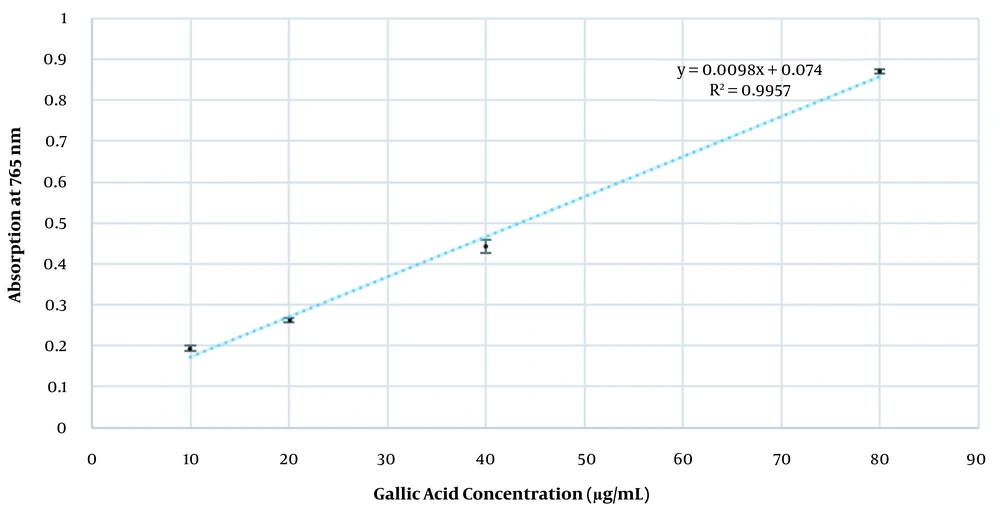
Total phenolic content (TPC) of the extract was quantified using a slightly modified version of the original Folin-Ciocalteu’s method (13). Triplicate concentrations of the standard gallic acid in 70% ethanol (10, 20, 40, and 80 µg/mL) were used to plot a calibration curve. One mL of the suitable concentration of the extract (500 µg/mL) was added to three test tubes. This concentration was found after trial and error to fit linear range of the calibration curve. Another tube was dedicated as the negative control, containing only 1 mL of 70% ethanol. Then, 2 mL of the Folin-Ciocalteu’s reagent (diluted 1:10 with deionized water) was added to the tubes. After 10 minutes, 1.5 mL of 7.5% (w/v) sodium bicarbonate was added to each tube. All tubes were incubated in the dark for 30 minutes. Finally, their absorption was measured at 765 nm using Optizen 2120UV Plus (Korea) spectrophotometer. The absorptions were put into calibration curve formula, and the TPC was expressed in terms of gallic acid equivalent (GAE) mg/g of the extract.
3.4. Preparation of Cream
The aqueous phase consisted of the plant extract, Tween 80, methyl and propylparaben, benzyl alcohol, EDTA, and deionized water, and the lipid phase comprised TiO2, Span 60, cetearyl alcohol, isopropyl alcohol, and isopropyl myristate. First, both phases were heated to 75 ± 1°C. A small portion of the aqueous phase was separated after reaching 45 ± 1°C and later added to the formulation to prevent possible decomposition of active compounds. The initial cream was prepared by the addition of the aqueous phase to the oily phase while being stirred at 1000 RPM using IKA RW 20 (Germany) stirrer. Next, the mixture was homogenized using MICCRA D-15 (Germany) homogenizer. Finally, Sepigel 305 was added to the mixture, and the finished cream was further stirred at 1000 RPM until it was cooled down to the room temperature.
3.5. Emulsion Type Determination
Emulsion type was determined by performing three tests. First, electrical conductivity was measured using the conductivity meter (Jenway 4070; UK), in which paraffin was used as control. In addition, a sample of cream was examined under a microscope (Zeiss, Germany) and was investigated for oil or water droplets. Finally, a dilution test was performed to confirm the findings of previous tests. In this test, a sample of cream was diluted using deionized water. The type of emulsion (water/oil or oil/water) was determined by assessing the solubility and homogeneity of cream in water.
3.6. Stability Studies
The finished product was subjected to accelerated stability tests at 40 ± 2°C/75 ± 5% RH for a total of three months. During this period, analysis of physicochemical parameters as well as microbial assessment and phenolic screening were performed upon manufacturing the cream and at 1st and 3rd months, subsequently.
3.7. Physical Parameters
The manufactured cream was examined for phase separation and changes in odor, color, homogeneity, and consistency. Moreover, pH, density, and viscosity were measured.
3.7.1. Determination of pH
The digital pH meter (Metrohm 827-Swiss) was used to measure the pH of the preparation. Examinations were repeated in triplicate. The pH meter was calibrated using standard buffer solutions (pH = 4 and 7) before use.
3.7.2. Density
The density of a substance is its mass per unit volume. The exact volume of pycnometer was measured by filling it completely with water, and the sides were lightly tapped to eliminate the air bubbles. The container was weighted full of a cream, and simply the density was examined.
p = m/V
p = density, m = mass, V = volume.
3.7.3. Viscosity
The cream viscosity was assessed by a Polyvisc Viscometer (Brookfield-USA) using a spindle R6 at 100 rpm. Viscosity measurements were carried out at room temperature (25 ± 2°C). All measurements were performed in triplicate.
Viscosity in centipoises (cps) = Dial reading × factor
3.8. Microbial Assessments
For microbial assessment, 10 g of cream was mixed with 3 g of sterile polysorbate 80; then, 24 g of 10% tween 60 water solution was added to the TSB medium to diagnose the bacteria (mixture 1). Plates with 25 - 250 colonies were calculated, and the average number of colony forming units (CFU)/g was counted. Two dilutions (0.1, 0.01) with the TSB culture medium were prepared, and 1 - 2 mL of pre-prepared dilution was added to 2 sterile plates containing 10 - 20 mL of TSA culture medium and incubated for 24 - 48 hours at 30°C - 35°C (pure plate). One mL of 0.1 dilutions for fungal counting allowed the agar to solidify at 25 ± 2°C, then incubated at 20°C - 25°C for 5 - 7 days. Colonies were counted after the incubation; all preparations were carried out in triplicate. All microbial counts were carried out via pour plate and incubated for 3 days, and then counting was performed. Counting was approved in plates containing fewer than 100 colonies for microbial count (TAMC) and in those comprising less than 10 colonies for fungal count (TYMC).
Salmonella Count: Colonies were grown in Rappaport Vassiliadis Salmonella Enrichment broth medium and counted after 18 - 48 hours of incubation at 30°C - 35°C.
Enterobacteriaceae Count: Colonies were counted after 18 - 24 hours of incubation at 30°C - 35°C.
Candida albicans Count: To achieve this aim, 10 mL of mixture 1 was added to 100 mL Sabouraud Dextrose agar (SDA) medium culture and incubated at 30°C - 35°C for 3 - 5 days. After growing the colonies, it was incubated for 24 - 48 hours.
Escherichia coli Count: Colonies were grown in MacConkey broth medium and counted after 18 to 72 hours of incubation at 30°C - 35°C.
Pseudomonas aeruginosa Count: Mixture 1 was grown in Cetrimide agar and incubated at 30°C - 35°C for 18 - 72 hours.
Staphylococcus aurous Count: After having any S. aurous growth in mixture 1, it was cultured in Mannitol Salt agar and incubated at 30°C - 35°C for 18 - 72 hours, and the colonies were counted.
3.9. Preservative Effectiveness Test
A standardized inoculum containing three bacteria (P. aeruginosa, E. coli, and S. aureus), a yeast (C. albicans), and a mold (Aspergillus niger) was mixed with the liquid product. The mixture comprising 0.05% of polysorbate 80 was added to adequate sterile buffer phosphate (pH = 7.2) to gain a count of about 1 × 108 CFU per mL. The absorption of the inoculum was seen at 580 nm. Then, 10 g of preparation was added to 90 mL buffer phosphate (mixture 2), and 0.1 and 0.01 dilutions of mixture 2 was prepared. Then, 1 mL of 0.1 and 0.01 dilutions was added to a sterile plate comprising 15 - 20 mL of the TSA medium, which cooled to 45°C, and incubated for 24 - 48 hours at 30°C - 35°C. The fungal process was alike to the above, excluding that the SDA culture with 0.1 dilution was incubated for 5 - 7 days at 20°C - 25°C. Positive and negative controls were used during the test. Microbiological examinations were performed for preparations at 25°C after 14 and 28 days of the formulation.
3.10. Quantification of Phenolic Content in the Finished Product
In order to extract active ingredients from the finished product, 1 g of the cream was weighed and added into 50 mL of 70% ethanol. The supernatant was removed three times by decantation using 50 mL of hexane. Finally, the supernatant was separated and concentrated using rotary evaporator. A concentration of 1000 µg/mL of extracted material was prepared each month, and 1 mL of it was used for quantification of total phenolic according to the above-mentioned method plus a negative control. The measurements were done in triplicates.
3.11. Statistical Analysis
Values in all experiments are represented as mean ± SD of at least three independent experiments. Statistical analysis was performed by repeated measurement analysis of variance (ANOVA) test. The significant level was also set as P < 0.05.
4. Results and Discussion
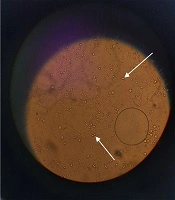
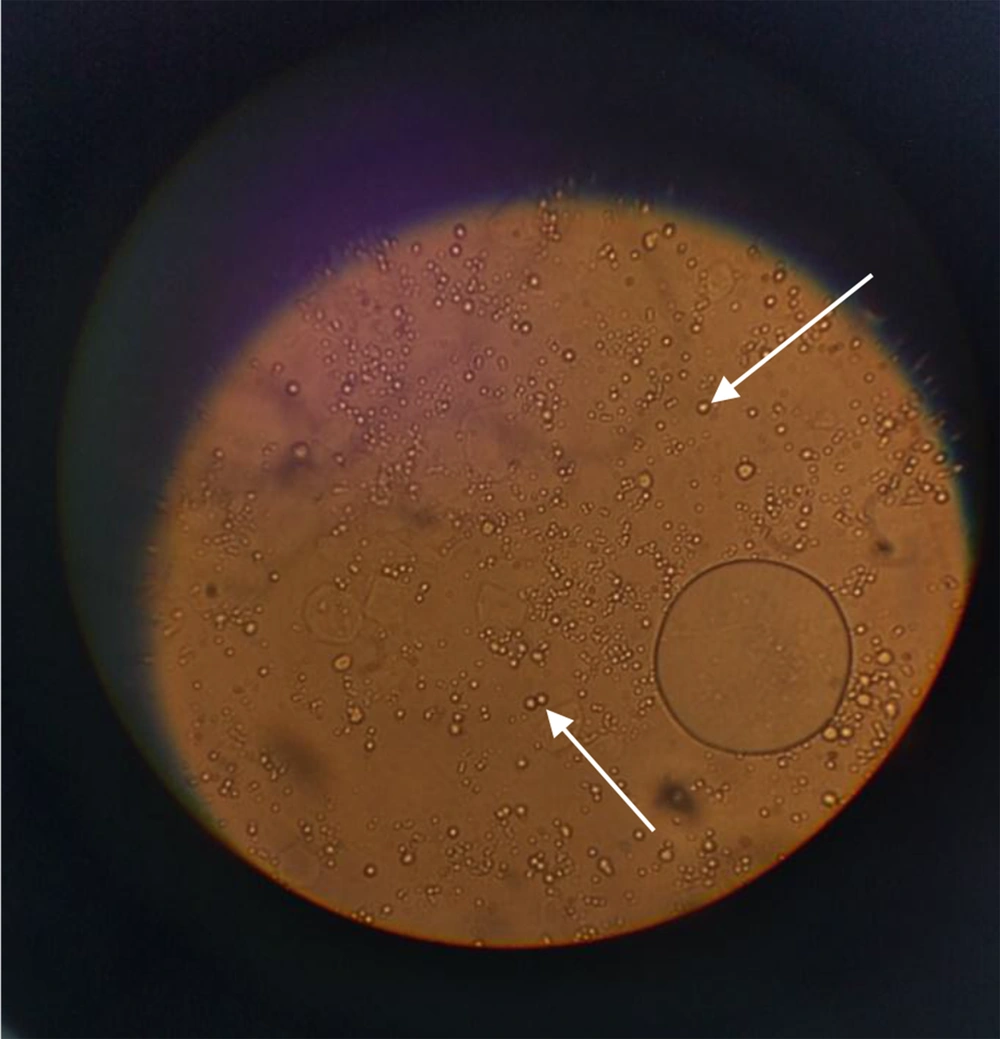
The extraction yield for 1 g of dried leaves was 3.4% for 96% ethanol and 7.5% for 70% ethanol. The extraction yield for the main maceration for 1 kg of the dried leaves was 34.3%. The extract was a dark green viscous liquid. The cream was light green in color and homogenous in texture upon preparation. The emulsion electrical conductivity was 0.33 millisiemens/cm, while paraffin showed zero electrical conductivity, indicating oil in water emulsion. The dispersion of oil droplets in the aqueous phase was recognized in the microscopic images (Figure 1).
The formulation showed no variation in color, odor, pH, and phase separation during the specified time span. The pH of the cream at the initial, 1st, and 3rd months was 6.3 ± 0.15, 6.05 ± 0.02, and 5.83 ± 0.037, respectively (Table 1). Viscosity at the initial, 1st, and 3rd months was 4240 ± 32.50 cP, 6810 ± 79.57 cP, and 7720 ± 40.51 cP, respectively. Lastly, the density of cream at the initial, 1st, and 3rd months was 0.98 ± 0.01, 0.95 ± 0.005, and 0.96 ± 0.01 g/cm3, respectively. Microbial assessment at the initial, 1st, and 3rd months showed less than 10 colonies for respective bacteria and fungi, which indicates adequate and efficient antimicrobial activity. The standard curve equation was y = 0.0098x + 0.074, where R2 = 0.9957 (Figure 2). There were no significant changes within stability time in assay, pH, density, and viscosity (P-value of all following physicochemical characteristics was greater than 0.05).
The total phenolic contents (mg.GAE/g of extract) in the cream at the initial, 1st, and 3rd months were calculated to be 24.90 ± 0.58 mg/g, 48.61 ± 1.38 mg/g, and 38.61 ± 1.13 mg, respectively (Table 1).
| Test | Period of Storage (40°C ± 2°C, 75% RH ± 5% RH) | Standard | ||
|---|---|---|---|---|
| Initial | 1st Month | 3rd Month | ||
| Description | Complies | Complies | Complies | Light green cream, homogenous cream mass |
| Odor | Complies | Complies | Complies | Characteristic order |
| pH | 6.3 ± 0.15 | 6.05 ± 0.02 | 5.83 ± 0.037 | 5-7 |
| Viscosity, cP | 4240 ± 32.50 | 6810 ± 79.57 | 7720 ± 40.51 | NLT* 4000, NMT** 10000 |
| Density, g/cm3 | 0.98 ± 0.01 | 0.95 ± 0.005 | 0.96 ± 0.01 | 0.90 - 1 |
| Total phenolic content, GAE mg/g | 24.90 ± 0.58 | 48.61 ± 1.38 | 38.61 ± 1.13 | |
| Microbial enumeration and tests for specific microorganisms | ||||
| Total bacterial count, CFU/g | < 10 | < 10 | < 10 | NMT 100 |
| Total fungi and yeast count, CFU/g | < 10 | < 10 | < 10 | NMT 10 |
| Pseudomonas aeruginosa | Neg. | Neg. | Neg. | Must be negative |
| Staphylococcus aureus | Neg. | Neg. | Neg. | Must be negative |
| Escherichia coli | Neg. | Neg. | Neg. | Must be negative |
| Salmonella spp. | Neg. | Neg. | Neg. | Must be negative |
| Candida albicans | Neg. | Neg. | Neg. | Must be negative |
| Antimicrobial Effectiveness test | Ok | Ok | Ok | Bacteria: NLT 2 log reduction from the initial count at day 14, and no increase from day 14 count to day 28. Yeast and Mold: No increase from the initial count at days 14 and 28. |
aAll experiments was performed in triplicate and written as a mean ± SD.
b*, Not less than; **, not more than.
The current body of evidence attributes anti-melanogenesis activity of the plant to its phenolic compounds. Thus, we manufactured a formulation using crude 70% ethanolic extract of leaves of Morus alba L. and analyzed its phenolic content and stability. Using crude extract provides a possible synergism between phenolic compounds as opposed to fractionating to a single compound. For sufficient extraction of phenolic compounds, we used 70% ethanol as the solvent; compared to 96% ethanol, it had a better extraction yield measured by dry weight of extracts. Since concentrated extract was quite viscous, adhesive, and staining, we tried oven drying to obtain a more suitable form, but it resulted in no significant weight difference, much less than 10%, so the concentrated extract remained without any changes. Our formula was an O/W cream which had a light and non-oily texture. The physicochemical properties of the cream, namely pH, viscosity, and density, were assessed, as well as total phenolic content screening using Folin-Ciocalteu’s method. All these properties were satisfactory. The O/W cream microbial status was also in accordance with the requirements of USP 40-NF 35 guidelines, including microbial enumeration tests of non-sterile products, tests for specified organisms, and antimicrobial effectiveness testing. All antimicrobial excipients were in the range according to the handbook of pharmaceutical excipients (14-16).
The concentrated extract itself was screened for total phenolic content as a reference point. The total phenolic content of the formulation surprisingly increased relative to initial measurement. Thus, the concentrated extract was screened again and compared to the reference point to make sure the prolonged period of storage had not decreased the total phenolic content. Again, the concentrated extract showed an increase relative to the initial reference measurement. The possible reasons are breakdown or hydrolysis of large polyphenols such as gallocatechin gallates into smaller phenols, alteration in interactions between excipients and phenolic components, and/or release of bound phenolic functional groups. Moreover, mixing and homogenizing the cream can cause further breaking of cellular structures since it was a crude extract, thus making reducing phenols more available for Folin-Ciocalteu’s reaction.
5. Conclusions
Hyperpigmentation treatment requires the long-term usage of topical drugs and current treatments face challenges as their aggressiveness and chronic use may cause side effects such as irritant dermatitis, exogenous ochronosis, and epidermal atrophy. Consequently, research has been carried out to find substitutes, herbal substitutes in particular. Different parts of the plant Morus alba L. has demonstrated promising whitening effects, which has been attributed to its phenolic compounds, such as oxyresveratrol and moracin, among many others. The crude extract from the leaves of this plant has never been formulated and undergone stability tests. Hence, in this study, we formulated and examined physicochemical properties of a cream based on the leaves of white mulberry. The manufactured formulation passed the respective stability tests, microbial assessment, and phenolic screening. In brief, the finished product passed the respective accelerated stability tests, and is therefore a promising formulation to be tested as a skin whitening agent.