1. Background
Glioma is the most prevalent type of the central nervous system’s cancers and accounts for more than 30% of all primary malignancies of the central nervous system. This cancer originates from glial cells, and the main cause of it is not known. Hereditary genetic disorders such as neurofibromatosis types 1 and 2 and tuberous sclerosis are known to predispose to glioma (1). Another predisposing factor for this cancer is cell phone electromagnetic radiation (2). Most brain tumors of the glioma type have been associated with cytomegalovirus infection, but the direct role of viruses in triggering this type of cancer has not yet been confirmed (3). Due to the invasive behavior of this tumor, the rapid proliferation of cancerous cells, and the lack of a proper treatment, this cancer causes many problems for the patient (4). Grade IV glioma, also known as glioblastoma multiforme (GBM), is the most malignant and progressive brain cancer and one of the deadliest human solid tumors, accounting for about 54% of malignant gliomas (5). Some GBM tumors arise from the development of pre-existing grade ΙΙ and ΙΙΙ astrocytomas (known as secondary GBM). However, about 90 to 95% of GBM cases develop de novo (i.e., primary GBM) (6). The risk of GBM increases with age, and men are about 1.6 times more likely than women to develop the disease. Also, this type of cancer is more prevalent among whites than other ethnic groups, and its rate is 3.44 cases per 100,000 people (6).
Temozolomide (TMZ), under the brand name of Temodar or temodal, is used as a standard oral chemotherapy drug to treat GBM (7). In cancer cells, DNA methylation is thought to be the main mechanism responsible for the toxicity induced by TMZ (8). Resistance to drugs is an important obstacle restricting the effectiveness of cancer drugs and is caused by some mechanisms such as drug inactivation, drug expulsion from tumor cells, repair of cellular destruction through chemotherapy, activation of survival pathways, and inactivation of cellular death pathways. The main effort to overcome therapy resistance is to use combinations of several drugs with minimal toxicities from different pharmaceutical groups (9). The standard treatments used for GBM, including surgical tumor resection, chemotherapy, and radiation therapy, generally fail to extend the short survival of patients after diagnosis. In fact, long-term survival is currently not accessible due to the innate and acquired resistance of the tumor to therapy.
Over the past millions of years, many natural products have been used to treat a variety of ailments despite the lack of scientific confirmation of their effectiveness and safety. Herbal remedies have been used by experimental physicians for centuries (10). Advances in technology have allowed scientists to identify the active ingredients of herbal extracts. Thus, efforts are underway to find new drugs to either complement or replace conventional therapies (11).
Harmine, a β-carboline alkaloid, was first separated from the seeds of Peganum harmala and Banisteriopsis caapi in 1847. It is extensively present in numerous medicinal herbs and has long been utilized in folk medicine in the Middle East and Asia. Previous research has shown the anticancer activities of harmine against different cancers, including lung (12), gastric (13), breast (14), and hepatic (15) cancer.
As previously mentioned, patients with GBM have a relatively short lifespan due to the low sensitivity of tumor cells to TMZ. On the other hand, increasing the concentration of the drug to boost its effectiveness causes very severe side effects.
2. Objectives
Due to the need to identify new effective therapies and enhance the efficacy of TMZ, this study aimed to investigate the effects of the combination of TMZ and harmine on the cell viability and invasion potential of T98G cells, a GBM cell line.
3. Methods
3.1. Cell Line and Reagents
The GBM cell line (T98G) was purchased from the Pasteur Institute of Iran. Harmine, TMZ, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), ethanol, crystal violet, trypsin, and dimethyl sulfoxide (DMSO) were provided from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Roswell Park Memorial Institute (RPMI) 1640 medium, penicillin/streptomycin solution, and fetal bovine serum (FBS) were procured from Gibco (USA). Stock solutions (200-mM) of TMZ and harmine were prepared in DMSO and diluted in the culture medium to reach final desired concentrations. The cells were seeded in the culture flasks containing RPMI 1640 supplemented with 10% FBS and 1% antibiotics (penicillin/streptomycin). The cells were maintained at 37°C in a humidified incubator under 5% CO2.
3.2. Viability Assay
The MTT assay was used to investigate the effects of TMZ and harmine on cell viability. The cells were cultured into a 96-well plate (1.5 × 104 cells/well) and incubated overnight. Then the cells were treated with different concentrations (1.56, 3.12, 6.25, 12.5, 25, 50, 100, and 200 µM) of TMZ and harmine in a serum-free medium for 24, 48, 72, and 96 hours. Next, the medium was discarded, and 50 μL of MTT solution (5 mg/mL in PBS) was added to the wells. After 2.5 h, to evaluate the rate of the reaction, 100 µL of DMSO was added. The plate was then placed on a shaker at room temperature for 20 min, and the absorbance was read by an ELISA reader at 570 and 630 nm (16). Cell survival percentage was determined according to the following formula:
The half-maximal inhibitory concentration (IC50) values of TMZ and harmine were calculated using GraphPad Prism software version 6.
3.3. Median Effect Analysis
The quantitative evaluation of the type of the interaction (i.e., synergistic, additive, or antagonistic) between TMZ and harmine was assessed using MTT viability measurement and data analysis by CompuSyn software, according to Chou and Talalay (17, 18). The combination index (CI) is an indicator of the type of the interaction between two agents. A CI value less than one is an indicator of synergism; a CI value equal to one shows an additive interaction, and a CI value greater than one reflects antagonism. Besides, the dose reduction index (DRI) was calculated. In this regard, a DRI value of less than one shows that using the combination of two drugs reduces the dose required for each when they are utilized alone. The combination of harmine and TMZ, with a constant ratio of 1: 2.66, was administered at two concentrations; above and below their IC50 values. The MTT test was performed again for the co-treatments. Using CompuSyn software, the plots of the effect vs. dose (Fa vs. dose), CI (Fa vs. CI), and DRI (Fa vs. DRI), as well as the normalized isobologram were drawn.
3.4. Migration Assay
The in vitro scratch test was used to evaluate the effects of TMZ and/or harmine on the cells’ migration ability. The T98G cells were cultured in the logarithmic phase of growth in 6-well plates (1.2 × 106 cells/well). After the cells reached a density of 80%, a scratch was made in the middle of the wells by a sterile yellow pipette tip. The wells were washed three times with PBS, and 1.5 mL of the culture medium containing 800 μM TMZ and/or 300 μM harmine was added. After 24 hours, the wells were evaluated and photographed under a light microscope (19). The images were analyzed by Tathcratch software (MathWorks Inc).
3.5. Invasion Assay
To evaluate the effects of TMZ and/or harmine on the cells’ invasion potential, the CytoSelect™ 24-Well Cell Invasion Assay kit (Cell Biolabs, San Diego, CA) was used. First, the inserts were placed in a plate in the presence of 300 μL serum-free medium in each well and incubated at 37°C for one hour. The hydrating medium was removed gently, and 500 μL of the medium containing 10% FBS was transferred to the lower chamber of the plate. The cells (7 × 105) pretreated with 800 μM TMZ and/or 300 μM harmine were cultured in the upper chamber for 24 h. Then the cells adhering to the surface inside the matrigel were removed by a swab. The inserts were transferred to a well containing 400 μL of the day solution and incubated for 10 min. Afterward, the inserts were gently rinsed with distilled water and dried. Finally, the inserts were transferred to an empty well, and 200 μL of the extraction solution was added to each well, and the plate was placed on a rotary shaker for 10 min. The absorption of the samples was measured at 560 nm. By comparing the absorption intensity of the treated samples with that of the control, changes in the invasion ability of the cells under the influence of the treatments were evaluated.
3.6. Adhesion Assay
For this assay, the wells of a 96-well plate were coated with a matrigel solution (5 mg/mL) and allowed to dry at room temperature for an hour. The cells exposed to 800 μM TMZ and/or 300 μM harmine for 24 h were trypsinized and suspended in the culture medium. Five hundred cells were cultured in each well and incubated for 24 h. Then the wells were washed with PBS, and the remaining cells were fixed with 4% paraformaldehyde solution for 20 min and then stained with 5% crystal violet for 10 min. To quantify connected cells, violet crystals were dissolved in 70% ethanol, and the absorbance of each sample was measured at 570 nm (20).
3.7. Gene Expression Analysis
The effects of TMZ and/or harmine on the expression of the genes encoding matrix metalloproteinase (MMP)-2 and 9 were assessed by real-time PCR. Total RNA from both control and treated cells was extracted by the TRIzol reagent (Thermo Fisher Scientific, MA, USA), and RNA quality was tested by determining the absorbance ratios of A260/280 and A260/230, and its integrity was assessed by agarose gel electrophoresis. Complementary DNA (cDNA) synthesis was performed utilizing 1 μg of the extracted RNA by a cDNA synthesis kit (Vivantis Technologies, Selangor DE, Malaysia) according to the manufacturer’s protocol. As an internal control, β-actin was used. Thermal cycles were as follows: 15 min at 50°C for cDNA synthesis and 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C to denature DNA and 45 sec at 60°C to anneal and extend the template for the PCR reaction. Real-time PCR was conducted by SYBR Premix Ex Taq Technology (Takara Bio Inc., Shiga, Japan) in the Applied Biosystems StepOne real-time PCR system. All primers were designed utilizing GeneRunner software, synthesized by CinnaGen Co. (Tehran, Iran), and checked at the NCBI Primer Blast. The primers’ sequences were as follows: (1) MMP-2: (F) 5’TTGGCAGTGCAATACCTGAA3’, and (R) 5’GAGTCCGTCCTTACCGTCAA3’; (2) MMP-9: (F) 5’CATCGTCATCCAGTTTGGTG3’, and (R) 5’CAGAAGCCCCACTTCTTGTC3’, and (3) β-actin: (F) 5’GTGGGCGCCCAGGCACCA3’, and (R) 5’CTCCTTAATGTCACGCACGATTT3’.
3.8. Statistical Analysis
All experimental tests were independently repeated at least three times. The data were presented as mean ± standard deviation (SD). Statistical analysis was conducted in SPSS version 16.0 software using one-way analysis of variance and Tukey’s post-hoc test, and differences were considered significant at P < 0.05.
4. Results
4.1. Cytotoxic Effects of TMZ and Harmine on T98G Cells
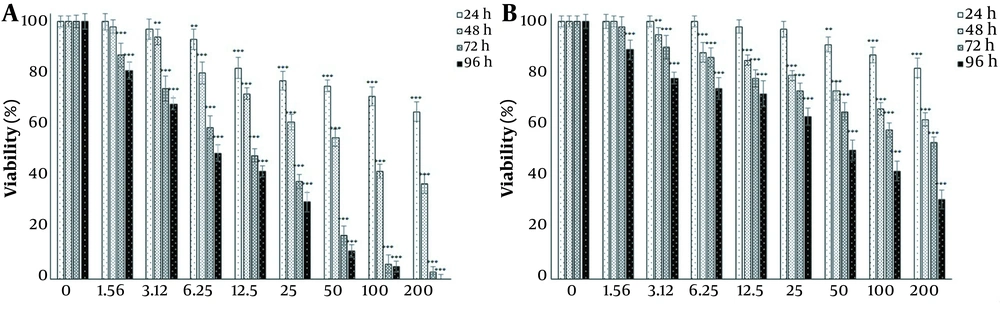
Since the main goal of this study was to investigate the potential of harmine in improving the toxicity of TMZ on T98G cells, first, the effect of each agent on cell viability was investigated independently. The effects of 1.56, 3.12, 6.25, 12.5, 25, 50, 100, and 200 μM doses of TMZ and harmine were investigated on cell survival and proliferation after 24, 48, 72, and 96 h of exposure (Figure 1A and B). The results revealed that both TMZ and harmine reduced the viability of the cells in a concentration- and time-dependent manner. Table 1 shows IC50 values.
A, Harmine; and B, temozolomide effects on the viability of T98G cells. Viability was evaluated by the MTT assay after 24, 48, 72, and 96 h of treatment with the 1.56, 3.12, 6.25, 12.5, 25, 50, 100, and 200 μM doses of temozolomide and harmine. The control group received the same volume of serum-free medium (* P < 0.05; ** P < 0.01; and *** P < 0.001 compared to the control).
| Variables | 24 hr | 48 hr | 72 hr | 96 hr |
|---|---|---|---|---|
| Temozolomide (µM) | 802.27 ± 3.85 | 341.11 ± 7.26 | 138.93 ± 3.71 | 44.75 ± 6.28 |
| Harmine (µM) | 316.74 ± 5.04 | 62.68 ± 4.57 | 9.82 ± 5.75 | 6.60 ± 5.10 |
a The values are presented as mean ± SD from three independent experiments.
4.2. Synergistic Effects of TMZ and Harmine on T98G Cells
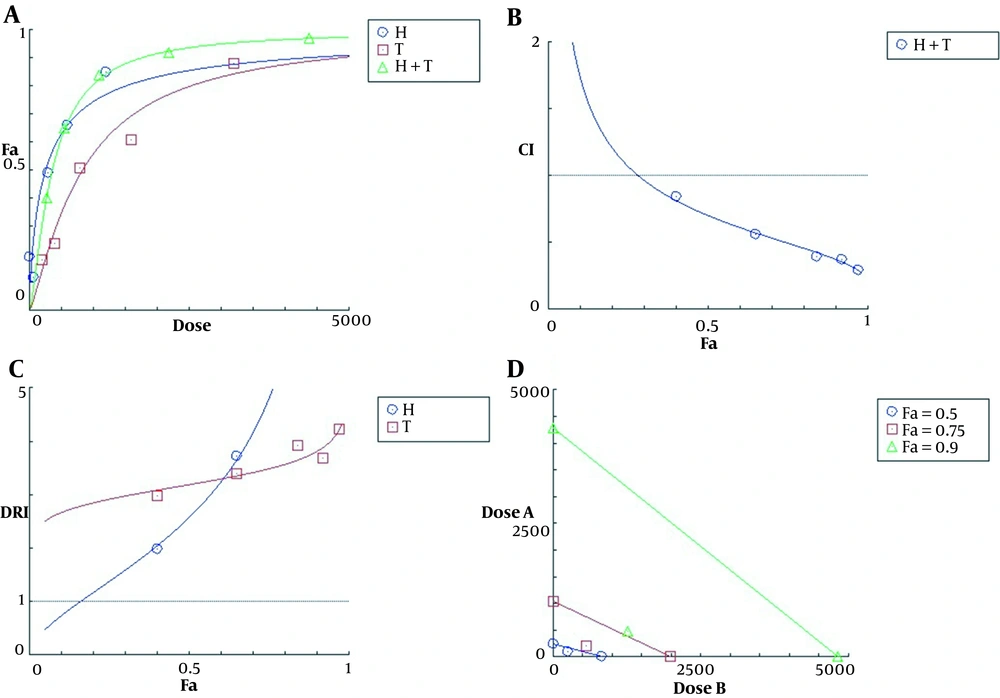
The effect-dose plot showed that the combination of the two agents was more toxic than each of them alone (Figure 2A). The calculated CI values for all five co-treatments were less than one, indicating a synergistic interaction between TMZ and harmine in reducing cell viability (Figure 2B). The DRI values of TMZ and harmine were greater than one, indicating a decrease in the dose required to produce a specific therapeutic effect in both cases (Figure 2C). Finally, the isobologram diagram was drawn (Figure 2D), in which the concentrations of TMZ and harmine, either alone and in combination, reducing the cellular population by 50, 75, and 90% were plotted. In this diagram, the localization of compound points on the chord, its bottom, and its top represented additive, synergistic, and antagonistic interactions, respectively.
The plots of A, effect-dose; B, combination index; C, dose reduction index; and D, isobologram for T98G cells after 24 h of treatment with temozolomide and harmine, alone and in combination with each other. Fa represents the fraction of the cells affected, and Fu represents the fraction of non-affected cells (i.e., treated and non-treated). The data were obtained using the MTT assay.
4.3. Inhibitory Effect of TMZ and Harmine on GBM cells’ Migration, Invasion, and Adhesion
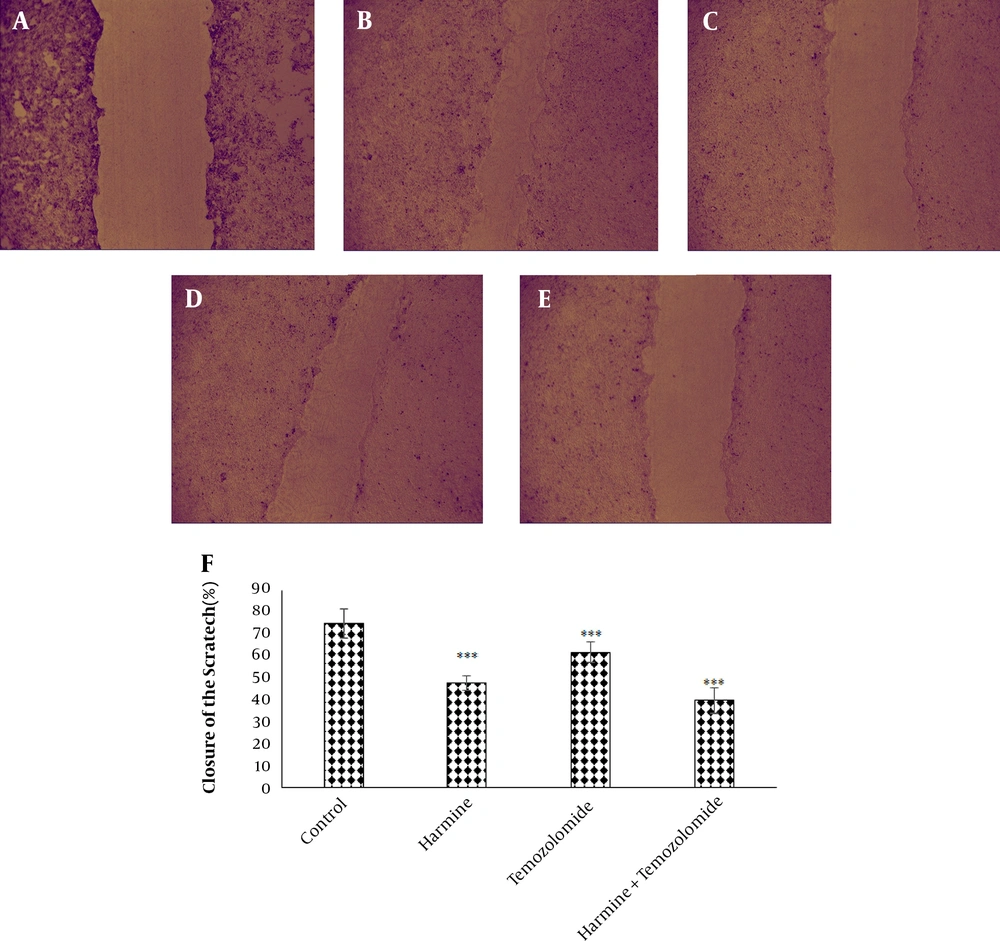
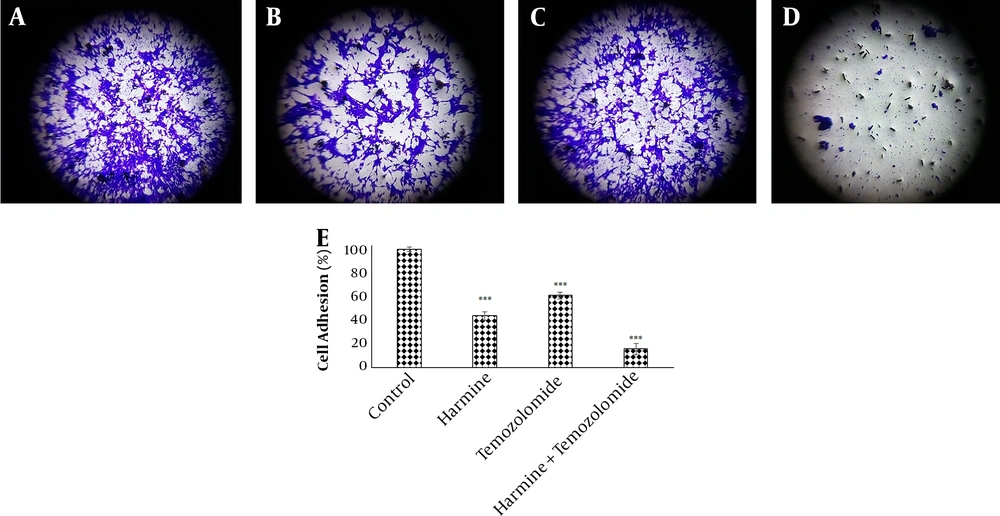
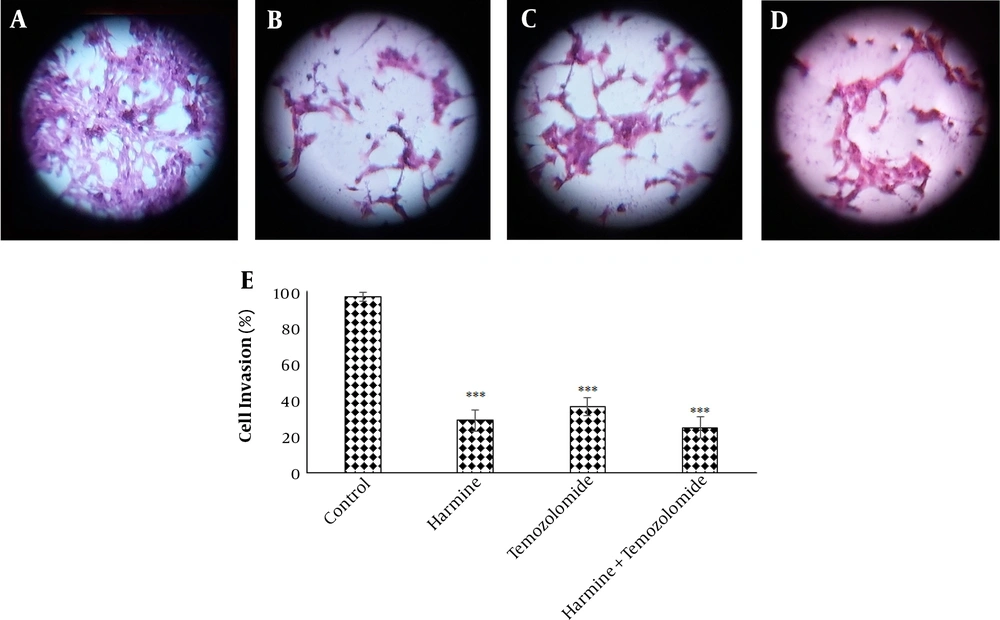
The analysis of the images obtained from the scratch test by Tscratch software showed that both TMZ and harmine decreased cellular migration capacity, with the combination of the two agents revealing a greater inhibitory effect than either alone (Figure 3). As shown in Figure 4, TMZ and harmine reduced cellular invasion potential, with a greater inhibitory effect in the combined compared to individual treatments. The results of the adhesion assay showed that TMZ and harmine reduced cellular adhesion, and the combination treatment had a greater inhibitory effect than either agent alone (Figure 5).
The effect of temozolomide and/or harmine on the migration ability of T98G cells was measured by the scratch test. The control group A, on day zero; B, after 24 h; C, in the presence of 300 μM harmine; D, in the presence of 800 μM temozolomide; and E, in the presence of a combination of the two agents; F, the column diagram of the average percentage of scratch closure in the T98G cell monolayer (* P < 0.05; ** P < 0.01; and *** P < 0.001 compared to the control).
Temozolomide and/or harmine effect on the invasion ability of T98G cells after 24 h was measured by the invasion test. A, the control group; B, in the presence of 300 μM harmine; C in the presence of 800 μM temozolomide; D, in the presence of a combination of the two agents; and E, the column diagram of the average absorption at 560 nm (* P < 0.05; ** P < 0.01; and *** P < 0.001 compared to the control).
Temozolomide and/or harmine effect on the adhesion ability of T98G cells after 24 h was measured by the adhesion test. A, the control group; B, after treatment with 300 μM harmine; C, after treatment with 800 μM temozolomide; D, after treatment with a combination of the two agents; and E, the column diagram of the average absorption of violet crystals at a wavelength of 590 nm (* P < 0.05; ** P < 0.01; and *** P < 0.001 compared to the control).
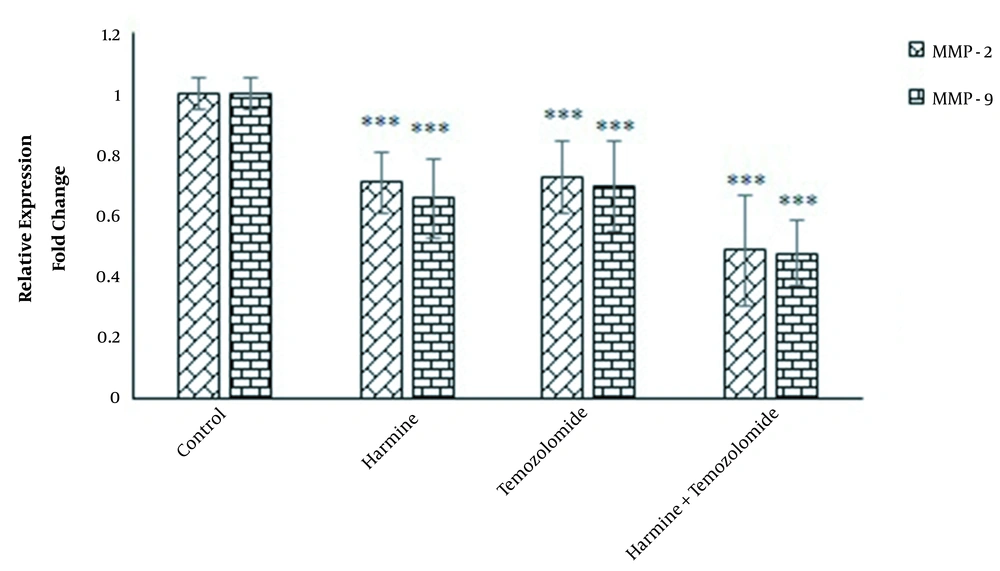
4.4. Effects of TMZ and Harmine on MMP-2 and MMP-9 Gene Expression in GBM Cells
Real-time PCR data revealed that both MMP-2 and MMP-9 were downregulated by TMZ and/or harmine (Figure 6).
5. Discussion
In this study, the effects of TMZ (an FDA-approved chemotherapy drug for GBM) and harmine (the main active ingredient of P. harmala seeds), both alone and in combination, were investigated on the cell viability of a GBM cell line. The results showed that at all doses of TMZ, cell viability gradually decreased in a concentration-dependent manner. After 24 h of treatment, cellular viability significantly reduced at the concentrations of 50, 100, and 200 μM compared to the control group. After 48 and 72 h of treatment, the effects of TMZ on cellular viability were significant at the 3.12, 6.25, 12.5, 25, 50, 100, and 200 µM concentrations, and after 96 h treatment, the cells’ viability significantly reduced at all concentrations. Also, harmine gradually decreased cell viability in a dose-dependent way. After 24 h of treatment, viability significantly diminished at the concentrations of 6.25, 12.5, 25, 50, 100, and 200 μM compared to the control. Following 48 h of treatment, the reduction in cellular viability was significant at concentrations of 3.12, 6.25, 12.5, 25, 50, 100, and 200 μM. After 72 and 96 h, all concentrations of harmine significantly reduced viability compared to the control group.
Previous in vitro and in vivo studies have demonstrated that harmine shows significant anticancer properties, including reducing cellular proliferation (21), migration (13), and invasion (22), as well as activating apoptosis (13) and preventing tumorigenesis. The cytotoxic effects of harmine have been noted against some cancers, such as gastric (23), lung (24), breast (25), and hepatic (26). It appears that harmine arrests the cell cycle at the G0/G1 phase (27), reduces cyclin-dependent kinase activity (28), induces autophagy and apoptosis, enhances the level of pro-apoptotic factors, and decreases the expression of pro-inflammatory cytokines (21). Harmine induces autophagy via upregulating LC3-II and down-regulating P62 (29) and also suppresses the expression of pro-metastatic genes such as MMP-9 and ERK, as well as vascular endothelial growth factors to reduce cancer invasion (30).
Despite recent advances in cancer treatment, there are still no significant improvements in GBM patients’ life expectancy and quality of lives, mostly because of drug resistance. Therefore, the development of new therapeutic strategies is necessary to overcome this problem. Recently, drug combinations have been widely used to treat fatal diseases such as cancer and the acquired immunodeficiency syndrome. The main goal of this strategy is to achieve a synergistic therapeutic effect, decrease drug dose and toxicity, and minimize or delay the development of drug resistance. In fact, synergistic interactions can reduce drugs’ toxicities and minimize the resistance of cancer cells against them. Today, the use of several anticancer drugs from different groups is widely applicable to treat various malignancies. According to research evidence, the combination of TMZ with other anticancer agents can boost its activity. For example, clinical studies have shown the beneficial outcomes of adding chloroquine to the standard GBM therapeutic regimen (31). Also, the combination of TMZ and carmustine, as a new adjunctive therapy in patients with GBM, showed satisfactory effects and tolerable toxicity (32). In vitro studies have also shown that the anticancer effects of TMZ are augmented in combination with some natural anti-tumor agents (33-42). However, no studies have been performed on the efficacy of TMZ + harmine co-treatment in suppressing the growth of GBM cells.
The results of this study showed that harmine significantly increased the cytotoxicity of TMZ, with a combination index between 0.28 and 0.84, indicating a synergistic effect between the two agents in the co-treatment state. The mean combination index for all the tests was 0.48, reflecting an overall synergistic effect for the combination of TMZ and harmine against the T98G cell line. This combination reduced the concentration of TMZ and harmine required for a specific therapeutic effect. Using this combination, the calculated IC50 values of harmine and TMZ reduced 1.22 and 2.54 times, respectively. Reducing the dose of TMZ for creating a certain effect is clinically valuable because it reduces the general side effects of chemotherapy. In this study, for the first time, the synergistic effects of TMZ and harmine on the viability of GBM cells were demonstrated after combined treatment. Our data also revealed that TMZ and/or harmine decreased the migration, invasion, and adhesion activities of T98G cells.
Cellular migration and invasion are major features of malignant tumors, especially in GBM. Although GBM cells cannot develop metastasis to other organs, their invasion and proliferation in the brain tissue are among the leading causes of death in these patients. A disseminated growth pattern is one of the characteristics of this type of tumor (43). Therefore, this ability to invade the brain, along with inherent resistance to TMZ, is the main barrier to the successful treatment of this disease, so managing this invasive behavior can be a useful strategy for effectively treating GBM.
As proteolytic enzymes, MMPs play important roles in the metastasis, migration, invasion, growth, and angiogenesis of tumors. These enzymes catalyze the decomposition of various components of the extracellular matrix. Among more than 20 members of this enzymatic family, MMP-2 and MMP-9 are of particular importance in GBM studies because their expression is directly related to the grade of the malignancy and its progression rate. The expression of both MMP-2 and MMP-9 is increased in human glioma tissues compared to the normal brain tissue, especially in GBM tumors (43). Since tumor cells’ ability to migrate across the intercellular matrix primarily depends on the secretion of MMPs, suppressing these enzymes can be a suitable therapeutic option (44). The results of our study showed that TMZ and/or harmine reduced the ability of T98G cells to migrate, adhere, and invade, accompanied by the downregulation of the genes of MMP-2 and MMP-9.
The results of previous studies have shown that concentrations below the lethal dose of TMZ reduce the invasive and migratory properties of GBM cell lines (45, 46). Also, harmine was shown to repress cellular migration and invasion in gastric cancer via reducing cyclo-oxygenase-2 gene expression and inhibiting angiogenesis and tumor growth by activating the p53 molecule in endothelial cells (13, 22).
5.1. Conclusion
According to the results of the present study, TMZ and harmine inhibited the cellular proliferation of a GBM cancerous cell line, with the combination of the two agents exerting a more prominent synergistic inhibitory effect. Harmine and TMZ also suppressed the migration, invasion, and adhesion capacities of GBM cells, and these effects were greater in the co-treatment state.