1. Background
Today, the development of new antifungal agents is in great demand due to the cases of antifungal resistance and the health risks related to the occurrence of fungal contamination of foods worldwide (1). Filamentous fungi are estimated to account for 50 - 75% of health-related problems due to the consumption of contaminated food (2). Harmful metabolites produced by filamentous fungi can be found in various food commodities throughout the food chain (3, 4). Fungal contamination reduces the nutritional value of foods and can cause both acute and chronic effects (3). Despite the development of new diagnostic and therapeutic procedures, filamentous fungi are still associated with high morbidity and mortality (5, 6). Among various types of filamentous fungi, Aspergillus, Fusarium, and Penicillium species have been described as the main causes of serious fungal contamination in cereals, grains, and fruits (4, 7).
Aspergillus spp. (A. ochraceus, A. niger, and A. flavus) belongs to a group of molds that have been reported as the main proportion of fungal contamination found in the food industry. Peanut, oilseeds, maize, sorghum, figs, cocoa beans, spices, rice, fruits, and vegetables are susceptible to contamination with Aspergillus sp. (1). Fusarium sp. is another group of molds that have a worldwide distribution (3). Wheat and maize are among the most important food crops susceptible to contamination with F. graminearum.
Due to the above-mentioned health risks and the high prevalence of fungal contamination in foods, researchers are increasingly looking for new targeted antifungal agents with maximum efficacy (2, 5). In this regard, natural resources have gained broad attention in the last two decades due to their promising effects for fungal inhibition with the least side effects (8). Fungi are one of the new spectrums of natural antifungal agents that have long been used for their pharmaceutical and health-promoting effects (9). Natural products and secondary metabolites derived from mushrooms have intense fungal inhibition (8).
Ganoderma lucidum (Curtis) P. Karst (also known as “Reishi” or “Ling Zhi”) is a well-known mushroom that has interesting pharmacological and nutraceutical properties (10, 11). The history of the therapeutic use of G. lucidum goes back to 4000 years ago. G. lucidum is a rich source of bioactive compounds such as polysaccharides, fatty acids, terpenoids, nucleotides, steroids, fatty acids, proteins, glycopeptides (12-14), phenolic compounds (11, 15, 16), and triterpenes (17). These bioactive compounds are mainly found in the fruiting body and the mycelium of this mushroom (18). Researchers have reported many health-promoting properties for G. lucidum, including antioxidant (10, 19, 20), anti-cancer (21, 22), anti-tumor, antimicrobial, anti-inflammatory, and cytotoxic activities (13-17, 23-25), cholesterol and hypertension control (13), and immune system promotion (12, 17). Also, several studies have shown the antifungal activity of G. lucidum against some harmful fungal species, including Aspergillus spp., Fusarium spp., Penicillium spp. (15, 26-29), and Candida albicans (30). Due to its significant properties, G. lucidum can be considered as a potential option for the production of new natural and healthy antifungal agents. Most of the antimicrobial effects of G. lucidum have been reported in bioactive compounds obtained from fruiting bodies and not from its mycelium (26). The high proportion of polyunsaturated fatty acids (17) and the diverse structure of bioactive polysaccharides contribute to the antimicrobial activity of G. lucidum (20). However, in a recent study, Arias-Londoño et al. reported that potential antifungal protein extracts are also responsible for the antifungal activity of G. lucidum against the phytopathogen fungus Mycosphaerella fijiensis (27). Also, Wang and Ng described the antifungal activity of Ganodermin, an antifungal protein from fruiting bodies of G. lucidum (31).
In this study, the fruiting bodies of G. lucidum isolated from the northern forests of Iran, Mazandaran province, were studied as a possible source of bioactive compounds that show antifungal activity against some filamentous fungal species, including A. flavus, A. ochraceus, and F. graminearum. This is the first attempt to evaluate the antifungal property of G. lucidum against F. graminearum.
2. Objectives
To determine the antifungal susceptibility of A. flavus, A. ochraceus, and F. graminearum to G. lucidum extracts.
3. Methods
3.1. Media and Chemicals
Sabouraud dextrose agar (SDA) and RPMI 1640 media were purchased from Merck, (Darmstadt, Germany) and BIO-IDEA (Tehran, Iran), respectively. Amphotericin B vial was acquired from Cipla Ltd., India. Tween 80 and normal saline (0.9% sodium chloride) were available for preparing fungal suspension. Ethanol 99.8% was purchased from Merck (Darmstadt, Germany). The commercial standardized aqueous powdered extract of G. lucidum with at least 30% polysaccharide content was prepared from Biocan Pharma (North York, Canada).
3.2. Apparatus
UV/Visible Spectrophotometer Cecil CE 1021, MA, USA was used for the optical density (OD) detection of the fungal suspension at 530 nm. Microplate reader Statfax 2100, Awareness Technology, Inc., FL, United States, was applied for the analysis of 96-well microplate absorption at 460 nm.
3.3. Preparation of Ganoderma lucidum
The fruiting bodies of G. lucidum were purchased as dry matter from Sarin Fam Company with the product no. AS-12, which was originated from Waz forest, Chamestan, Noor city, Mazandaran Province, Iran.
3.4. Preparation of Ganoderma lucidum Extracts
G. lucidum extracts were prepared by the cold maceration method. First, 10 g of dried grounded G. lucidum fruiting bodies was carefully weighed, and 200 mL of each solvent (sterile distilled water, ethanol 99.8%, and ethanol/water 60:40) was added into a 500-mL bottle with a screw cap covered with an aluminum foil. The ethanolic and hydroalcoholic extracts were kept at room temperature for two weeks. The bottles were occasionally shaken by hand and visually checked for any cross-contamination. The two extracts were filtrated twice through a Whatman filter paper no. 1.
The aqueous extract was shaken for 24 h by an orbital shaker stirrer at 150 rpm, and then it was filtered twice by a Whatman paper no.1, and the extract was kept at 40°C for further investigations. The hydroalcoholic and ethanolic extracts were concentrated close to dryness at a controlled temperature (40°C) by a rotary evaporator, and the concentrated extract was dissolved in dimethyl sulfoxide (DMSO) to achieve the desired concentrations (9).
3.5. Preparation of Fungal Strains
Three fungal strains, including A. flavus, A. ochraceus, and F. graminearum, were purchased from Pasteur Institute of Iran (PII) with pathogenic fungi culture collection (PFCC) no. 50041, 401-10, and 573, respectively. All the three fungal strains were sub-cultured on sterile SDA medium at 30°C for seven days.
Then, seven-day-old colonies were covered with 5 mL of sterile solution (100 mL saline normal + 10 µL tween 80) and were gently probed with the tip of a transfer pipette for the preparation of the suspensions. Next, the mixture containing conidia and hyphal fragments was transferred to a sterile tube and allowed to settle for 3 to 5 min. Then, the resulting upper suspension was transferred to a sterile tube and mixed with a vortex for 15 s (32).
Sporangiospore suspensions were prepared by a spectrophotometric procedure, and it was found that viable conidial concentrations in the range of 0.4 - 5 × 104 CFU/mL had the most reproducible minimum inhibitory concentration (MIC) data.
The spectrophotometric method was applied for the OD measurement of the microbial suspensions. The OD for the genus Aspergillus should be adjusted to 0.09 - 0.13 and for Fusarium 0.15 - 0.17 (32).
3.6. Antifungal Assays
3.6.1. Preparation of Concentrations
The extracts were filtered through 0.45-micron syringe filters and were diluted to make eight desired concentrations (5, 4, 3.5, 3, 2.5, 2, 1.5, & 1 mg/mL).
The concentrations of amphotericin B for A. flavus and F. graminearum were prepared as 0.004, 0.002, 0.001, and 0.0005 mg/mL (5, 32); and for A. ochraceus as 0.032, 0.016, 0.008, and 0.004 mg/mL (33).
3.6.2. Minimum Inhibitory Concentration Assay
The MIC was measured according to the clinical laboratory standards institute (CLSI) broth microdilution method (M38-A2) for filamentous fungi (32). First, 100 µL of RPMI1640 culture medium was added to each well. Then, each concentration was inoculated into a round-bottomed 96-well microplate by adding 100 µL of the fungal suspension until the final volume reached 200 µL. A well-known antifungal agent, amphotericin B, with different concentrations (6 replications) was considered as the positive control, and the culture media plus DMSO were used as the negative control. Also, the microplates were incubated at 35°C for 48 h (32).
3.6.3. Minimum Fungicidal Concentration Assay
The minimum fungicidal concentration (MFC) Assay values for all the G. lucidum extracts were measured by removing 10 μL of the contents of the wells with no visible growth and pouring them on SDA plates. Then, the plates were incubated at 28°C for 72 h. The visible growth of the fungi was inspected to find out whether G. lucidum extracts could completely inhibit the fungal growth at the MIC and above. The lowest concentration without any growth was reported as the MFC value (5).
3.6.4. Microplate Assay
The microplates were tested by visual inspection with the scores set by the CLSI method from 0 to 4 (0-no growth; (1) minor growth or around 25% of the growth control; (2) considerable reduction in growth or around 50% of the growth control; (3) minor reduction in growth or around 75% of the growth control; and (4) no reduction in growth) (32). Also, the absorbance spectrum of the wells was measured by a plate reader at 460 nm.
4. Results
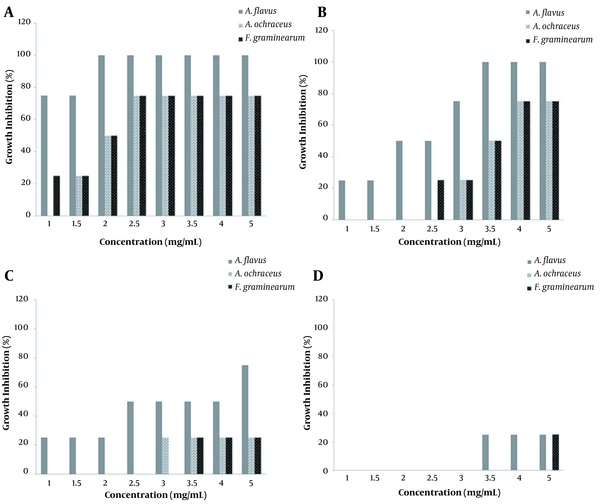
The results of antifungal effects of different extracts of G. lucidum are displayed in Figure 1. The growth of fungi was evaluated in growth inhibition percent. As observed, the ethanolic extract (A) was more effective than others with an MIC value of 2 mg/mL for A. flavus and this concentration for the other fungi was considered minimum inhibitory concentration required inhibiting the growth of 50% of organisms (MIC50 ). The growth of A. ochraceus and F. graminearum from 2.5 - 5 mg/mL was below 25%. At the lowest concentration (1 mg/mL), only A. ochraceus could completely grow without any visible reduction in fungal growth.
The hydroalcoholic extract of G. lucidum was found to be effective in the growth of A. flavus with the MIC level of 3.5 mg/mL. In addition, the same MIC50 was obtained for A. ochraceus and F. graminearum (Figure 1B). A. ochraceus was fully resistant to the hydroalcoholic extract below 2.5 mg/mL and F. graminearum at 2 mg/mL, while the growth of A. flavus was inhibited even at 1 mg/mL (< 25%).
The three studied fungi were resistant to the aqueous extract at all concentrations. However, the growth of A. flavus at higher concentrations was prevented poorly by the extract as observed in Figure 1C.
One of the applied extracts for the evaluation of antifungal activity was the commercially prepared aqueous extract as a powdered form which was compared with the aqueous extract prepared in this study. The results shown in Figure 1D imply that the commercial aqueous extract had a higher antifungal efficiency than the aqueous extract. As expected, this antifungal activity was weaker for A. ochraceus and F. graminearum, but the growth of A. flavus was moderately prevented. Therefore, the MIC value of 2.5 mg/mL can be considered as MIC50.
The results regarding the MIC, MFC, and MIC50 values of G. lucidum extracts are presented in Table 1. The ethanolic and the hydroalcoholic extracts showed promising potency for antifungal activity for concentrations between 2 - 3.5 mg/mL. However, both aqueous extracts did not have a satisfying effect. Although all the three fungi were equally sensitive to the alcoholic extract with an MIC value of 2 mg/mL, A. flavus was found to be the most sensitive fungal strain with the MFC value of 4 mg/mL. For the hydroalcoholic extracts, all the three fungal strains were equally sensitive, with an MIC value of 3.5 mg/mL. Again, only A. flavus was the most sensitive strain with the MFC value of 6 mg/mL. Moreover, no significant growth inhibitory pattern was found for the aqueous and commercial aqueous extracts. As can be noted in Table 1, the MICs of amphotericin for A. flavus, A. ochraceus, and F. graminearum were identified at 0.002, 0.004, and 0.002 mg/mL, respectively. Also, the MFC values obtained for these three fungal strains were 0.004, 0.008, and 0.004 mg/mL, respectively. As expected, the antifungal activities of amphotericin were several times stronger than MIC and MFC values obtained for G. lucidum extracts.
| Fungal strain | Extracts | ||||
|---|---|---|---|---|---|
| Ethanolic | Hydroalcoholic | Commercial Aqueous Powder | Aqueous | Amphotericin B | |
| Aspergillus flavus | ND | ||||
| MIC | 2 | 3.5 | 0.002 | ||
| MFC | 4 | 6 | 0.004 | ||
| MIC50 | 2.5 | ||||
| Aspergillus ochraceus | ND | ||||
| MIC | 0.004 | ||||
| MFC | 0.008 | ||||
| MIC50 | 2 | 3.5 | |||
| Fusarium graminearum | ND | ||||
| MIC | 0.002 | ||||
| MFC | 0.004 | ||||
| MIC50 | 2 | 3.5 | |||
The MIC, MFC and MIC50 Values (mg/mL) of Different Extracts of Ganoderma lucidum Against Filamentous Fungi (N = 6)
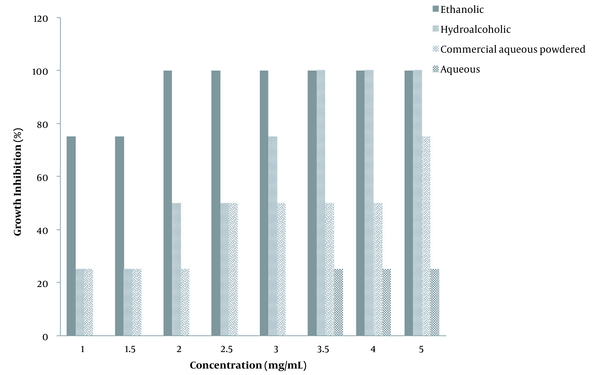
The growth inhibitory effect of the extracts on A. flavus is shown in Figure 2. As shown in Figure 2, the ethanolic extract at a concentration of 2 mg/mL was able to completely stop the growth of A. flavus. Also, the hydroalcoholic extract with a concentration of 3 mg/mL was able to 100% inhibit the growth of this fungus. However, both the commercial aqueous extracts and the aqueous extract did not achieve acceptable results, and only the commercial aqueous extract was able to inhibit the growth of the fungus at a concentration of 5 mg/mL up to 75%. The higher antifungal susceptibility for concentrations 1 - 5 mg/mL could be related to the increased alcoholic portion of the extraction phases. All the extracts could partially prevent the growth of A. flavus, and the sensitivity of this fungal strain to G. lucidum was obviously noted.
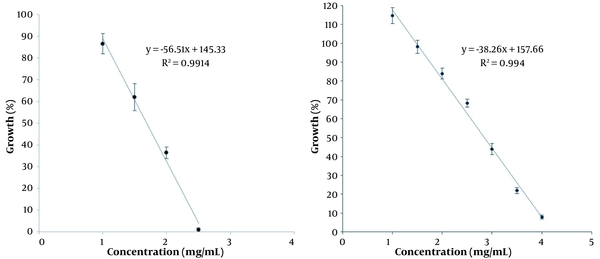
The microplates were analyzed by a plate reader at 460 nm. Figure 3 displays the effect of the ethanolic and hydroalcoholic extracts based on absorption data against A. flavus. The points in the graphs show that MICs in the ethanolic extract in plate reader data are about 2 and 2.5 mg/mL. Also, the hydroalcoholic extract was about 3.5 and 4 mg/mL.
5. Discussion
Ganoderma lucidum has many useful properties that are mentioned in traditional medicine references. One of these effects is antimicrobial activity against viruses, bacteria, fungi, and yeasts. The possible sources of antifungal activity for G. lucidum have been attributed to proteins (27), lipopolysaccharides (11), organic acids (15), and glycoproteins (34). The results related to antifungal properties of different parts of G. lucidum are inconsistent, and this difference can be attributed to the method of extraction and the type of harmful fungal strains.
Most studies have focused on pathogenic (15, 16, 35, 36) and food-contaminating fungal strains which are related to human health risk (9, 12, 37). However, the results of these studies are mixed. In our research, some scientific and novel points are considered for antifungal activity. G. lucidum was isolated from the northern forests of Iran and was investigated for the first time for its antifungal properties. Although Aspergillus species and its antifungal susceptibility to G. lucidum has been previously studied (15, 16, 35, 36), to the best of our knowledge, no valid study has been conducted on the antifungal properties of G. lucidum against F. graminearum.
The results showed that the ethanolic extract was more effective on the studied fungal strains compared to the other two extracts at the same concentrations, which were similar to the recent studies on alcoholic, ethanolic (35, 36), and methanolic (15, 16) extracts. The hydroalcoholic extract showed less effective antifungal properties than the ethanolic extract, but it was more efficient than the aqueous extracts. The ethanol/water ratio (60:40) that used in the hydroalcoholic extract was in compliance with the MIC results of the alcoholic and hydroalcoholic extracts, which is due to the fact that antifungal properties were because of compounds extracted in the alcoholic phase. Regarding the studied fungal strains, an interesting finding was that A. flavus was more sensitive to the extracts and amphotericin B compared to the two other fungi.
The designed procedure for the assessment of MIC in the aqueous extract of the mushroom was a slightly different than other studies. The extract was directly used for the preparation of the tested concentrations. Besides, the commercial standardized aqueous powdered diluted with the culture medium (RPMI) was tested in this study. The results demonstrated that A. ochraceus and F. graminearum were resistant to the applied concentrations of both extracts, but A. flavus showed moderate sensitivity to the commercial aqueous powdered extract at higher concentrations that could be considered as MIC50. The aqueous extract had no significant antifungal effect on any of the three studied fungi.
Regarding the MIC and MFC values reported in this study, it should be noted that although various studies have been performed on the antifungal properties of G. lucidum against our fungal strains, but these studies vary significantly in terms of test method, type of extraction (and consequently in terms of units reported for the final results), solvent type (ethanol, methanol, etc.), and fungal part used (mycelium, fruiting body, etc.), which makes it very difficult to compare the results and reach a firm conclusion (12). Although microdilution methods generally yield more accurate results compared to other methods (37), few studies have studied antifungal activity of G. lucidum against harmful food-contaminating fungal strains with this reference method. For example, MIC and MFC values reported by Heleno et al. (15) for the methanolic extract of G. lucidum against A. ochraceous were less than half the values reported in our study.
Also, three comparative studies were conducted on G. lucidum extracts obtained from different countries. In the first study, the MIC values reported by Stojkovic et al. (16) for G. lucidum methanolic extracts obtained from Serbia and China against A. ochraceous were 0.15 and 0.10 mg/mL, and the MFC values were 0.30 and 0.15 mg/mL, respectively. These values are significantly lower than the values obtained in this study. Similarly, Cilerdzic et al. (35, 36) conducted two studies on G. lucidum strains obtained from Serbia, China, and Montenegro and compared their results with a commercial strain. In these studies, different MIC and MFC values reported for G. lucidum ethanolic extracts against A. flavus ranged between 0.67 - 1.67 mg/mL and 2.67 - 3.33 mg/mL, respectively, which is almost comparable with the results obtained in our study. Finally, although studies on the antifungal properties of G. lucidum against F. graminearum have yielded promising results, these studies differ in terms of test method and unit of final result reported. In addition, although no study has been performed on F. graminearum, the results reported for other Fusarium species generally indicate that G. lucidum extracts are effective against this fungal species (31, 38, 39).
Significant differences in the obtained results limit the generalizability of our findings. These variations could be due to some possible reasons. The first one could be the geographical distribution of G. lucidum strains, which show different antifungal activities against similar fungal strains (16, 35, 36). Another reason may be the differences between the reference methods employed for testing antifungal susceptibility (40). Another possible reason may be the differences in extraction procedures as extraction parameters such as time, and particle size can influence the total phenolics content of G. lucidum ethanolic extract, which is thought to be effective on final antifungal activities of G. lucidum. In addition, the content of G. lucidum polysaccharides as a possible source of G. lucidum antifungal activity strongly depends on extraction parameters (11, 20).
5.1. Conclusions
Ganoderma lucidum is a medicinal mushroom with antifungal properties against harmful filamentous fungi isolated from food products. In this study, the antifungal properties of the ethanolic, hydroalcoholic, commercial aqueous powdered, and aqueous extracts of G. lucidum against the fungal strains of A. flavus, A. ochraceus, and F. graminearum were investigated by the broth microdilution method. Although the results obtained for the aqueous extract were not very promising, but the results related to the alcoholic extract may be useful in the food industry, and the alcoholic extract of this fungus can be used as a suitable alternative to chemical fungicidal drugs common in the food industry.
In the future, more diverse extracts and different solvents can be studied. Secondly, more research can be done on the reasons of the antifungal properties of this fungus to determine how this fungus can be best used in the food industry as an antifungal agent. In the future, different parts of the fungus can be tested to see if the antifungal properties of G. lucidum are different for different parts. Finally, the toxin production inhibition of G. lucidum against filamentous fungi can be investigated in future studies.