1. Context
Recently, green consumerism has been widely accepted, leading to the substitution of synthetic chemicals used in foods with natural compounds. It has significantly affected research studies, so that increased attention has been paid to applying natural preservative agents in foods. Various edible compounds are available for food packaging, including proteins and polysaccharides (1). Herein, many proteins have been used in food packagings, such as whey and soy protein, zein, gelatin, collagen, etc. (2). They have been used in the form of edible films to improve the shelf life and maintain the quality of various food products. Whey protein (WP) are of the best proteins used for this purpose due to its auspicious attributes. Some of the desirable features of WP-based films are being a well gas barrier and its glossy appearance, which makes them excellent for use in the food packaging industry (3). Moreover, other naturally occurring active compounds can be incorporated with WP-based films to achieve longer shelf life for foods.
Among all modern methods (4), a combination of nanotechnology and food packaging has manifested a new horizon to fulfill the production of healthy food with extended shelf life. One of the major impacts of this combination on food was yielded by the utilization of nanoscale antimicrobial and/or antioxidant agents in packaging. The application of these compounds with nanosized characteristics not only has intensified their beneficial effects but also improved their durability in food matrices (5). The potent antimicrobial and antioxidant effects of nanosized materials provided a new approach to address food quality issues as well as spoilage of food (6). This review aims to draw the attention of researchers and inspire the scientific community to barge in the application of WP-based films in foods. This study has reviewed the incorporation of nanoscale bioactive agents in the films as well as considered their impacts on the quality and shelf life of foods.
2. Evidence Acquisition
2.1. WP-Based Films with Antibacterial Effects
Whey protein is of the major proteins of milk, which is a by-product in some dairy processes. It can be recovered by new methods such as ultrafiltration and has been used for various purposes like the development of active food packaging (7). Various WP films that contain antimicrobial agents have been developed. For this purpose, many bioactive agents with antibacterial activity have been added to the WP-based films, for example, essential oils (EOs), TiO2, nanomaterials, etc. Antibacterial effects of these fabricated films are investigated against Gram-negative and -positive bacteria such as Escherichia coli, Pseudomonas fluorescens, Salmonella enteritidis, Listeria monocytogenes, and Staphylococcus aureus (8, 9). The antimicrobial activity of WP-based films is provided in (Table 1).
| Film Ingredients | Bioactive Compound | Method of Fabrication | Main Findings | |
|---|---|---|---|---|
| 1 | WPI/cellulose nanofibers | Rosemary EO (1.5 and 2%) and/or titanium dioxide (0.5, 1 and 1.5%) | Casting/evaporation method | Strong antibacterial effects (approximately 15 mm inhibition zone for Escherichia coli, Salmonella enteritidis, Pseudomonas fluorescens, Staphylococcus aureus, and Listeria monocytogenes), particularly against Gram-positive bacteria -Moderate antioxidant effect (Lower than butylated hydroxytoluene (BHT)) (8) |
| 2 | Cellulose nanofiber (CNF)/polydextrose/WPI | L. plantarum | Homogenization-sonication/casting | Growth inhibition of E. coli (12.6 ± 0.5 mm), S. aureus (14.0 ± 1.0 mm), and Pseudomonas aeruginosa (14.6 ± 0.5 mm) (10) |
| 3 | WPI/chitosan nanofiber | Cinnamon EO (4%) | Homogenization-sonication/casting | Antibacterial effects toward a Gram-positive (S. aureus) and Gram-negative (P. aeruginosa and E. coli) bacteria (11) |
| 4 | WPI | Water soluble derivative of chitosan (1%) | Casting | Excellent antifungal effects; 100% inhibition growth of Aspergillus niger (12) |
| 5 | WPI | Curcumin (0, 0.2, 0.5, and 1.0 mg/mL) | pH-driven | Excellent antioxidant effect (13) |
| 6 | WPI | Grammosciadium Ptrocarpum Bioss. essential oil (0.5, 1, 1.5%) | Casting | Antibacterial effects against Gram-negative (Salmonella typhimorium, E. coli, P. aeruginosa) and a Gram-positive (L. monocytogenes) bacteria. (9) |
| 7 | WPI/corn zein protein | TiO2/SiO2 | Sonication/casting | Antibacterial effect against E. coli. (14) |
| 8 | WPI/sodium caseinate/glycerol | Nano- TiO2 | Casting | Antibacterial properties toward S. aureus (12.01 ± 1.01 mm) and E. coli (14.40 ± 0.77 mm) (15) |
| 9 | WPI | Nano-clays (5%) | Sonication/solution casting | Antibacterial effects against; L. monocytogenes. (16) |
| 10 | WPI | Lavandula angustifolia EO (0.5, 1, 1.5, and 2%) | Homogenization-sonication/casting | Antibacterial effects toward Gram-negative and Gram-positive bacteria: S. aureus (16.17 mm), Bacillus subtilis (14.75 mm), E. coli (11.03 mm), and Salmonella enterica (11.98 mm) (17) |
| 11 | WPI | Silver nanoparticles (0, 0.25 and 1.25 mM) | Casting | Antimicrobial activity against yeast Williopsis saturnus (13.3 mm), mould Aspergillus sydowii (16.00 mm), and bacteria: S. aureus (15.00 mm), S. enteritidis, E. coli (13.3 mm), and L. monocytogenes (14.7 mm) (18) |
| 12 | WPI/cellulose nanocrystal | rambutan peel extract (3%) | Casting | Strong antioxidant effects; Medium antibacterial effect (19) |
| 13 | WPI | Coconut EO (0, 0.4, 0.8% w/v) and paprika extract (0, 0.03, 0.06% w/v) | Homogenization-sonication/casting | Significant antioxidant properties; Antibacterial effect (20) |
| 14 | WPI/gelatin/inulin | Lb. curvatus 54M16 (4% v/v) | Casting | Significant antibacterial effects against pathogens due to the production of bacteriocin. (21) |
| 15 | WPI | TiO2 nanoparticles (10 and 15%) | Casting | Antimicrobial effects against E. coli (20 mm), Salmonella spp (19 mm), Pseudomonas aeuroginasa (18 mm), S. aureus (25 mm), Bacillus spp (22 mm), Candida albicans (23 mm). (22) |
| 16 | WPI | Lemon and bergamot EO (4.5%) | Casting | Better antimicrobial properties of bergamot EO against E. coli (21 mm) and S. aureus (20.5 mm) -Bergamot EO, also showed antifungal activity against A.niger (35 mm) (23) |
| 17 | WP concentrate | Thyme | High pressure-thermal treatment | Antimicrobial effects against Torulopsis stellata, Geotrichum candidum, and B. subtilis. (24) |
| 18 | WPI/nanocellulose | Nanoemulsion of bergamot EO (5, 10, 20, 40%) | Casting | Antibacterial activity toward E. coli (21 mm), S. aureus (15 mm), L. monocytogenes (21 mm), P. aeruginosa (19.5 mm) (25) |
| 19 | WPI/chitosan | Combination of TiO2 (1 and 2%) and ZEO | Casting | Antibacterial effects toward Gram-negative and Gram-positive bacteria: E. coli (9.5 mm), S. aureus (23.2 mm), and L. monocytogenes (8.5 mm) (26) |
| 20 | Guar gum/sago starch/WPI | Carvacrol and citral | Casting | Appropriate antibacterial effect on B. cereus and E. coli (27) |
| 21 | WPI | Citrus sinensis peel EO (2.5 and 5%) | Casting | Antioxidant properties (28) |
| 22 | WPI | Lignin microparticle (0, 0.25, 0.50, and 0.75% w/w) | Casting | Antioxidant activity (29) |
| 23 | WPI | Natamycin and nanoemulsion of α-tocopherol | Casting | Antioxidant activity; Antimicrobial activity toward Penicillium chrysogenum (288.2 mm), C. albicans (150.9 mm), and Saccharomyces cerevisiae (303.5 mm), respectively (30) |
| 24 | WPI/starch | Rosemary EO | Homogenization-sonication/casting | Antioxidant activity (31) |
Abbreviations: WP, whey protein; WPI, whey protein isolate; EO, essential oil.
Among all compounds incorporated with WP-based films, EOs are the most important antimicrobial agents, such as cinnamon or rosemary. Mohammadi et al. (11) developed a biodegradable film using WPI, chitosan nanofiber, and cinnamon essential oil. They reported that the addition of cinnamon to film significantly increased its antimicrobial activity against Gram-negative (E. coli, P. aeruginosa) and -positive (S. aureus) bacteria. In another study by Ghadetaj et al. (9), WPI and nanoemulsion Grammosciadium Ptrocarpum EO were utilized to form a film. The antimicrobial test showed that the above-mentioned film had an antibacterial effect against Gram-negative and Gram-positive bacteria, and L. monocytogenes was the most susceptible bacteria. Ghadetaj et al. (9) also analyzed the antioxidant activity of the film and demonstrated the best antioxidant effects for the film containing 1% nanoemulsion G. Ptrocarpum EO. Lavandula angustifolia EO also was added to WPI film, and its antibacterial functionalities were investigated against S. aureus, Bacillus subtilis, E. coli, and Salmonella enterica (17). The results obtained by Rashed et al. (17) study showed that fabricated film was effective on Gram-positive and -negative bacteria. However, Gram-positive bacteria were more susceptible to the mentioned film. In addition, TiO2 has been used along with rosemary EO (8) and (26) Zataria multiflora EO in WP-based protein, and a summary of these studies was presented in Table 1. Other EOs and compounds that have been added to the WPI-based film (14-16, 22, 23, 25, 27) can also be found in Table 1. Overall, the addition of EOs to WP-based films grants them excellent antimicrobial effects and makes them suitable for active food packaging.
2.2. Whey Protein-Based Films with Antioxidant Activity
Whey protein-based films have been fabricated exclusively to achieve antioxidant effects. EOs and plant extracts are classified as major natural compounds (32), which are added to the whey protein-based films to increase the antioxidant activities of the bioactive compounds. The antioxidant activity of whey protein-based films is provided in Table 1. Taghavi Kevij et al. (13) used WPI and curcumin to develop a film with antioxidant effects. They reported that WPI film without curcumin showed slight antioxidant activity while adding curcumin dramatically increased (P < 0.05) the antioxidant activity of the film. Amjadi et al. (28) fabricated WPI films consisting of Citrus sinensis peel EO in the forms of emulsion and nanoemulsion that the results showed antioxidant activity of the film. Another study developed WPI/starch film embedded with rosemary and recorded antioxidant activity by DPPH analysis (31).
In addition, other natural materials originated from plants have been used to grant beneficial effects to the whey protein-based films. For instance, Sukatta et al. (19) used 3% rambutan peel extract (RPE) to fabricate a WPI/cellulose nanocrystal film with antimicrobial and antioxidant. The obtained data by the study of Sukatta et al. (19) revealed that film imparts remarkable antioxidant activity (even better than BHT and α-tocopherol) and mediocre antibacterial effects toward S. aureus. Moreover, the results of the addition of paprika extract and coconut EO (20), microparticles of lignin (29), and α-tocopherol (30) to WP-based films are outlined in Table 1.
2.3. Application of Whey Protein-Based Films in Food Models and Their Impacts on an Index of Food Quality
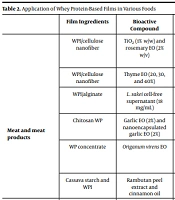
The most important factors affecting food quality, which are conducive to spoilage, are microbial deterioration and the oxidation process. A wide variety of antimicrobial and antioxidant agents has been added to whey protein-based films to prevent the growth of bacteria in food. These compounds can be divided into two categories of chemical and natural agents. Among natural bioactive compounds, EOs are the most frequent antimicrobial and antioxidant agents used to prevent food spoilage and pathogenic bacteria. Due to the simultaneous antimicrobial and antioxidant properties of EOs, they have been drawn much attention in research studies (33). The application of whey protein-based films in various foods is provided in Table 2.
| Film Ingredients | Bioactive Compound | Food Model | Main Findings | |
|---|---|---|---|---|
| Meat and meat products | WPI/cellulose nanofiber | TiO2 (1% w/w) and rosemary EO (2% w/v) | Lamb meat | Antimicrobial activity toward Enterobacteriaceae, Pseudomonas spp, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, and lactic acid bacteria; Better antibacterial effect against Gram-positive bacteria; Increased shelf life from 5 days to 15 days. (34) |
| WPI/cellulose nanofiber | Thyme EO (20, 30, and 40%) | Ground meat | Excellent antioxidant activity; Preserved the natural red color of meat; Prolong the shelf life (35) | |
| WPI/alginate | L. sakei cell-free supernatant (18 mg/mL) | Fresh beef slices | Antimicrobial properties against major pathogenic bacteria of beef; 2.3 log10 CFU/g reduction for E. coli; 1.4 log10 CFU/g reduction for L. monocytogenes; Ineffective on sensory characteristics (36) | |
| Chitosan WP | Garlic EO (2%) and nanoencapsulated garlic EO (2%) | Vacuum-packed sausage | Reduction of microbial growth (aerobic plate count, psychrophilic count, lactic acid bacteria count); Reduction of lipid oxidation; Increased shelf life; Ineffective on sensory properties (37) | |
| WP concentrate | Origanum virens EO | Traditional Portuguese sausages (paínhos and alheiras) | Reduced lipid oxidation; Inhibited the microbial growth; Increased the shelf life for 15 and 20 days for alheiras and paínhos, respectively (38) | |
| Cassava starch and WPI | Rambutan peel extract and cinnamon oil | Salami | Antioxidant and antimicrobial activity of film; Delayed the microbial deterioration (39) | |
| Poultry meat and poultry products | WPI | Oregano or clove EO | Chicken breast | Inhibition of bacterial growth (mesophilic and psychrophilic count, Enterobacteriaceae, and lactic acid bacteria count); Increased the shelf life (40) |
| WP concentrate | ethanolic extract of seaweed (Fucus vesiculosus L.) | Chicken breast | Lower malonaldehyde; Lower lipid oxidation; Increased shelf life (41) | |
| WP/chitosan | Quince and cranberry juice | Turkey meat pieces | Antimicrobial activity toward pathogenic bacteria (E. coli, Salmonella typhimurium, and Campylobacter jejuni); Reduced total bacterial count; Increased the shelf life for 6 days (42) | |
| WPI | Nisin, grape seed extract, ethylenediamine tetraacetic acid (EDTA), and malic acid | Turkey frankfurter | Antibacterial effects against pathogenic bacteria (E. coli, L. monocytogenes, Salmonella typhimurium) (43) | |
| Fish and fish products | WPI | Tarragon EO (2.5%) | Brook trout | Lower TBARS values, indicating antioxidant effects; Lower amounts of bacteria in the total viable count, lactic acid bacteria, psychrophilic count, and H2S producing bacteria, indicating antibacterial effects; 3 days of increased shelf life (44) |
| WP | Sodium alginate (0.5%) | Kilka | Antioxidant activity; -Lower amounts of TBARS, peroxide value, free fatty acid, pH, and TVN; Antimicrobial activity (reduction of total bacterial count) (45) | |
| WP | Anise oil (4 and 6%) | Dried Decapterus maruadsi | Antimicrobial and antioxidant effects; Increased shelf life from 3 to 21 days; Application of 4% anise oil in WP film showed no undesirable effects on sensory attributes (46) | |
| Vegetables and fruits | WP/pectin film | Transglutaminase | Apple, carrot, and potato | Increased the shelf life by at least 6 days.; Excellent antimicrobial and antioxidant activity (47) |
| WP | Olive oil | Peanut | Increased the shelf life; Improved the sensory attributes (48) | |
| WP/montmorillonite | Citric acid | Fresh-cut apple | Significantly increased the shelf life. (49) | |
| Chitosan/WPI | Citric acid | Strawberries | 40 and 60% increased shelf life at 20 and 5ºC. (50) |
Abbreviations: WP, whey protein; WPI, whey protein isolate; EO, essential oil.
2.3.1. Meats
So far, the effects of whey protein-based films in different food products have been investigated to assess their beneficial effects on shelf life. Meat and meat products are of the most important parts of human nutrition with high nutritional contents. Despite, rapid deterioration of these foods is quite challenging since they provide an excellent environment for microbial growth (51). Usually, meats contain considerable amounts of fat that makes them susceptible to rancidity. On the other hand, meat can be contaminated with spoilage and pathogenic bacteria during slaughter (52).
Some studies have been carried out to preserve their quality and increase their shelf life by employing whey protein-based films. Alizadeh Sani et al. (34) developed a film using WPI/cellulose nanofiber incorporated with TiO2 and rosemary EO and investigated its antibacterial activity as well as its effects on the shelf life of meat. The film showed a significant antibacterial effect, especially on Gram-positive bacteria. It was also associated with an increased shelf life of meat, from 6 days (control group) to 15 days. Carvalho et al. (35) focused on meat oxidation spoilage and tried to prevent lipid oxidation in ground meat using WPI/cellulose nanofiber films incorporated with thyme EO. They reported that the chosen film not only could retard the lipid oxidation of ground meat samples but also preserved its natural red color. The film can be used to increase the shelf life of meats with high lipid content. Moreover, the application of WPI films has been used not only for spoil retardation but also for the prevention of pathogenic bacteria in meat.
Other meat products, namely processed meat, have also been treated with whey protein-based film. Catarino et al. (38) developed a WP concentrate film embedded with Origanum virens EO to improve the physiological properties and microbial quality of two types of Portuguese sausages. Catarino et al. (38) demonstrated that WP-based film significantly reduced the lipid oxidation and microbial growth of sausage samples. In addition, the shelf life of sausages was significantly increased between 15 to 20 days. There are other studies that investigated the application of whey protein films to improve the shelf life of sausages. Esmaeili et al. (37) added garlic EO and nonencapsulated garlic EO to WP and chitosan edible film. They applied this film on the sausage to increase their shelf life during storage for 50 days. The data showed significant antimicrobial effects of fabricated films on aerobic plate count, psychrophilic count, and lactic acid bacteria count (P < 0.05). Thiobarbituric acid reactive substances and peroxide value analysis revealed the antioxidant activity of films. They also reported that WPI and chitosan films incorporated with nano-encapsulated garlic EO were ineffective on sensory properties of sausage samples which is important from the consumers’ acceptance aspect.
2.3.2. Poultry Meats
Poultry meats are another important meat product for human nutrition with a high rate of spoilage. Andrade et al. (41) formulated a film made of WP concentrate incorporated with ethanolic extract of seaweed (Fucus vesiculosus L.) and applied it to increase the shelf life of chicken breast. The results showed lower malonaldehyde content in the group treated with the chosen film, indicating lower lipid oxidation and better shelf life. Other efforts have been made to maintain the original quality of chicken stored in cold condition. Fernandez-Pan et al. (40) fabricated WPI films incorporated with oregano or clove EO and investigated its effects on chicken breast samples. Both films with oregano or clove EO were effective and exhibited antibacterial effects. However, oregano EO incorporated with WPI film was stronger. Both films were effective in microbial populations and increased the shelf life of chicken samples. The beneficial effects of the application of WP-based films on other poultry meat products such as turkey frankfurter have been evaluated by Gadang et al. (43). They made a WPI film incorporated with different compounds including nisin, grape seed extract, ethylenediamine tetraacetic acid, and malic acid, and assessed its antibacterial effects on turkey frankfurter inoculated with S. typhimurium, E. coli O157:H7, and L. monocytogenes. Promising bacterial results were observed for samples stored at 4ºC for 28 days, and the authors suggest the application of chosen film for prevention of foodborne diseases in these types of ready-to-eat products.
2.4. Fish
Seafood and fish were of the major segments of the human diet with excellent nutritious value. These foods are economically important, and many countries and areas around the world are dependent on them. Despite that, these foods are extremely susceptible to deterioration, which causes major economic losses every year. Many approaches have been used to retard seafood spoilage and prevent economic damages such as chilling, freezing, application of preservatives, etc. Recently, WP-based films have been applied by researchers to achieve this goal. Socaciu et al. (44) formulated a film using WPI and tarragon EO and evaluated its effects on quality indices of brook trout stored at 4ºC for 15 days. TBARS analysis revealed lower values for fish samples treated with the chosen film, which refers to its antioxidant activity. In addition, antimicrobial activity for the above-mentioned film was observed by microbial tests. For example, the total viable count for fish samples showed a significant reduction in log10 CFU/g which led to 3 days prolonged shelf life. Administration of psychrophilic count, lactic acid bacteria count, and H2S producing bacteria has resulted in similar results. Overall, the application of the WPI-based film incorporated with 2.5% tarragon EO could increase the shelf life by 3 days. WP-based films have also been employed on dried fish. Matan (46) formulated a WP film incorporated with anise oil and evaluated its impact on dried Decapterus maruadsi stored at 30ºC for three weeks. Findings of the study displayed that the film containing 4 and 6% anise oil exhibited antimicrobial and antioxidant characteristics and increased the shelf life of dried fish from 3 to 21 days (concerning different chemical and microbial analysis). They also investigated the undesirable effects of the mentioned film on sensory attributes, and it turns out that incorporation of 6% anise oil in WP film caused an unpleasant impact on dried fish. Overall, the study suggested that 4% anise oil embedded with WP-based film was the best choice to simultaneously increase the shelf life and did not affect the sensory characteristics.
2.5. Fruits and Vegetables
Fruits and vegetables are perishable foods that are quite challenging for industries due to the high spoilage rate. Some studies attempted to preserve these products using WP-based films. Rossi Marquez et al. (47) developed whey protein/pectin edible films by adding transglutaminase to increase the shelf life of apple, carrot, and potato. They reported that the excellent antimicrobial and antioxidant activity of the film caused increased shelf life of these foods for at least 6 days. In another study on fresh-cut apples by Azevedo et al. (49), WP/montmorillonite films are fabricated using citric acid, and the results indicated a significant increase in the shelf life. Muley and Singhal (50) embedded citric acid into chitosan/WPI edible films and investigated their effects on the shelf life of strawberries at 20 and 5ºC. The results showed a 40 and 60% increase in the shelf life of samples for 20 and 5ºC, respectively. WP-based films could also increase the shelf life of other foods such as peanut (48), tomato (53), grapes (53), etc.
3. Conclusions
In this review, the functionality of different applications of WP-based films to increase the shelf life of various types of foods was discussed. It has been proved that WP films can retard the lipid oxidation and rancidity of foods. The antimicrobial activity of WP films could inhibit the growth of spoilage and pathogenic bacteria such as S. aureus, E. coli, S. typhimurium, etc. The potent antimicrobial and antioxidant properties of WP-based films led to the shelf-life prolongation of different food products. Although, their application in foods confronts some challenges. For example, the incorporation of some bioactive compounds with WP films affects the sensory characteristics of foods. However, nanotechnology and encapsulation of bioactive compounds almost resolved this issue. Nanotechnology also intensified the beneficial effects of WP films, namely, antioxidant and antimicrobial effects. Nanotechnology approaches were also conducive to the slow release of bioactive compounds into the food matrix, and their antioxidant and antimicrobial effects were demonstrated for more duration. Overall, few studies have investigated the effects of WP films on food, and more research is required to extend our knowledge beyond the practical application of these films in food industries. In principle, this literature review confirmed the ability of edible WP-based films incorporated with different bioactive compounds in preserving the original quality of foods and increasing their shelf life. We suggest that more studies should be carried out on food model systems to ultimately achieve the best practical results in food industries. Moreover, the application of nanotechnology for the development of WP-based films is highly recommended to achieve the best results. Novel EOs with excellent in vitro antimicrobial and antioxidant effects also can be used for incorporation with WP-based films. Herein, some limitations and challenges must be considered before the application of WP-based films on an industrial scale. For example, the appearance, permeability, mechanical strength, effect on organoleptic quality, and so on. Another important issue that must be scrutinized is the investigation of the possible toxicity of WP-based films–to ensure the safety of consumers.