1. Background
The new infectious disease triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread broadly and emerged as a complicated illness that can remain asymptomatic or cause modest symptoms such as clusters of acute respiratory illness signs like fever, cough, dyspnea, pneumonia, etc. (1-3). Also, one-fifth of the hospitalized cases develop Acute Respiratory Distress Syndrome (ARDS) and require intensive care unit (ICU) treatments (4). While multiple strategies such as social distancing, self-isolation, and contact tracing might be prospective to achieve control of SARS-CoV-2 transmission, still a growing number of more than 182.3 million people with confirmed diagnoses of and more than 3.9 million deaths from the disease have been currently reported (5-7).
On the other hand, owing to the lack of proven drugs for the treatment of the proceeding coronavirus disease 2019 (COVID-19) pandemic, novel effective formulations of pharmaceuticals are in major attention (8). Fortunately, several groups dedicated themselves to achieving anti-SARS-Cov-2 modalities worldwide, of which herbal compounds and traditional medicine have been widely developed (9-11). These formulations indispensably played a vital role in the prevention and treatment programs for COVID-19 management, especially in China and South Korea, with a combination of modern medicine where they reduced the prevalence of the disease and mortality (9, 12, 13).
Reviewing the literature for formulations of traditional medicine and initial understanding of the COVID-19 disease, the authors formulated a novel herbal compound. Being a simple way for drug delivery and the most desired choice of the patients (14, 15), this formula, which was composed of 18 herb components and is being patented in the Iranian Patent Office (IPO) with an application number of 139950140003008767, was prepared as compressed tablets. The tablets are composed mainly: Terminalia chebula, Glycyrrhiza glabra, Anacyclus pyrethrum, Senna alexandrina, Ferrula asafoetida, Pistacia lentiscus, Zizyphus jujuba, Crocus sativus, Echinacea angustifolia, and Hyssopus officinalis. Moreover, we made use of other additional and complementary herbal derivatives and pharmaceutical excipients according to the traditional and modern medicine principles to make stable and uniform tablets. The herbal ingredients were selected due to their host-directed regulation and certain antiviral effects, which is stated by the available data in the Pharmacognosy science and traditional medicine (16-19).
2. Objectives
The current open-label, single-blind, randomized clinical trial was intended to scrutinize the efficacy and safety of novel oral pharmaceutical tablets with an innovative formulation of herbal ingredients. We sought to test whether the formulation is capable of altering the length of hospital stay (LoS), intensive care unit (ICU) admission, and mortality in patients with a confirmed diagnosis of COVID-19.
3. Methods
3.1. Study Design and Participants
Before starting the study, by referring to the literature and reviewing the available articles and books, the plants used in the management of viral triggered diseases such as severe acute respiratory syndrome (SARS), influenza, COVID-19, etc., especially in China and Korea, were selected and matched to the compounds and native plants of Iran botany and, finally, we presented a new plant composition. The most important and active ingredients of the tablets were Terminalia chebula, Glycyrrhiza glabra, Anacyclus pyrethrum, Senna alexandrina, Ferrula asafoetida, Pistacia lentiscus, Zizyphus jujuba, Crocus sativus, Echinacea angustifolia, and Hyssopus officinalis. Table 1 summarizes the details of the main active ingredients of this herbal compound and the antiviral, anti-inflammatory, and immunomodulatory effects thereof. Due to the warm nature of the composition, using the resources and references of Iranian traditional medicine, supplementary herbal ingredients had been added to the formulation to modify the nature. Also, common pharmaceutical excipients (lubricant, binders, etc.) were utilized in tablets’ composition to make tablets with appropriate physicochemical properties (e.g., hardness, erosion, dissolution rate, and stability).
| Number | Plant Name | Antiviral Effects, (Target Virus) | Anti-Inflammatory Effects (Ref.) | Immunomodulatory Effects (Ref.) | Parts Used for anti-SARS-CoV-2 Effects (Ref.) |
|---|---|---|---|---|---|
| 1 | Terminalia chebula | Dengue virus (16) | (20) | (21, 22) | Plant extracts (23, 24) |
| 2 | Glycyrrhiza glabra | H1N1 (25, 26), HCV (27), HSV-1 (28) | (29, 30) | (31-33) | Root (34) |
| 3 | Anacyclus pyrethrum | Common cold (35) | (36) | (37, 38) | Root (39, 40) |
| 4 | Senna alexandrina | HSV-1 (41) | (42) | (43-45) | Leaf (46) |
| 5 | Ferrula asafoetida | HSV-1 (47) | (48, 49) | (50, 51) | Root, Leaf (52) |
| 6 | Pistacia lentiscus | HSV1 (53, 54), HSV-2 (55) | (56, 57) | (58, 59) | Root, stem, leaf (60) |
| 7 | Zizyphus jujuba | Influenza A (61) | (62, 63) | (64, 65) | Fruit (66, 67) |
| 8 | Crocus sativus | HPV (68) | (69, 70) | (69) | Stigma, style (67, 71) |
| 9 | Echinacea angustifolia | Common cold (72) | (73) | (74) | Root, stem, leaf (75) |
| 10 | Hyssopus officinalis | HIV (76) | (77) | (78) | Dry weight of aerial parts (79) |
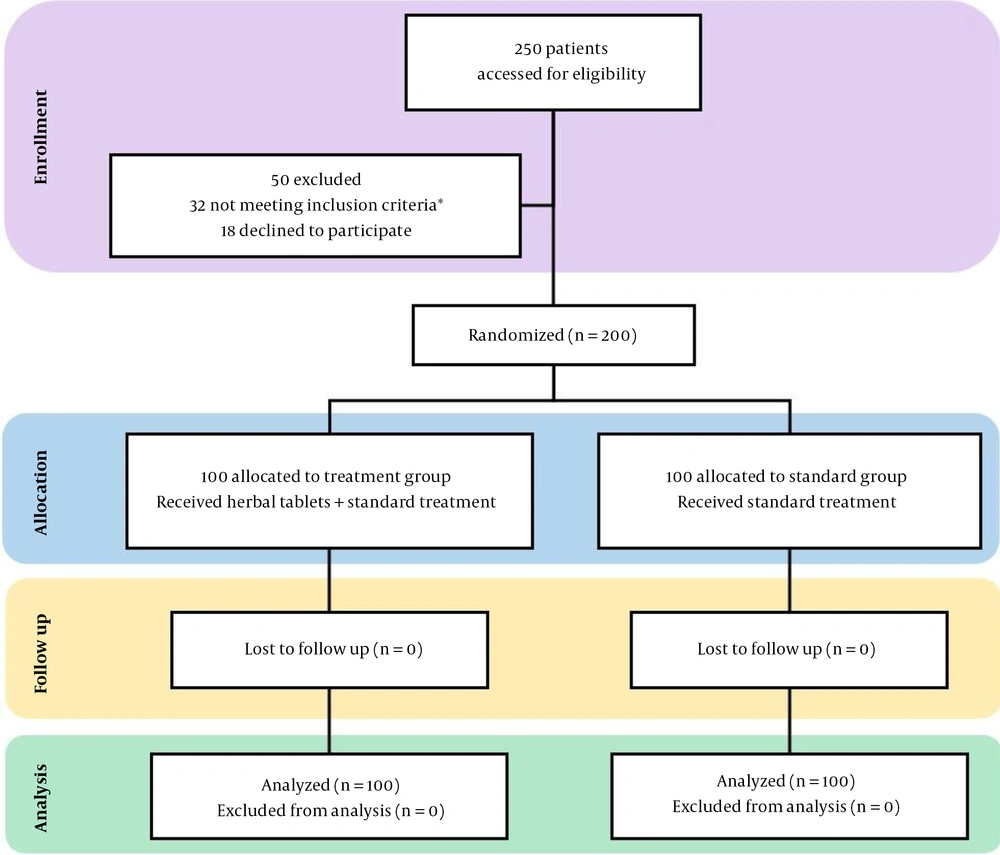
This randomized, open-label, single-blind, superiority clinical trial was done at Imam Reza (PBUH) hospital in Tabriz, northwest of Iran. Two hundred COVID-19 patients aged 18-65 years with tendency to attend a clinical trial study signed the informed consent and were randomized to either the treatment group (standard treatment based on the Iranian national COVID-19 treatment protocol (i.e., remdesivir, favipiravir, hydroxychloroquine, and supportive oxygen treatment) along with the compressed herbal tablets) or the control group (only received the standard treatment) in a 1:1 ratio, i.e., 100 subjects in each arm. Usage of all other common medications, such as corticosteroids, bronchodilators, etc., was almost the same in both groups. The incidence of the adverse reactions within 180 days after the beginning of the intervention was set as the safety endpoint, and a deliberate delay for 180 days in reporting the results was set to monitor the last patients for possible adverse effects.
The inclusion criteria were being aged 18 to 65 years, positive PCR or CT scan findings based on COVID-19 disease, willingness to participate in a clinical trial study, and disease severity in the mild to the moderate range with the following symptoms: low-grade fever (around 37.7 degrees Celsius), dry cough, fatigue, headache, new loss of taste or smell, gastrointestinal upset, including vomiting and diarrhea and/or itchy, painful patches on the skin.
The key exclusion criteria were namely severe COVID-19 disease status, liver or kidney failure, organ transplantation experience, pregnancy or breastfeeding, and, finally, receiving any clinical trial medication in the last 30 days before the enrolment.
3.2. The Extraction Method and Standardization of the Formula
The composition of the plants, after grinding into a fine powder, was formulated in the form of compressed oral tablets following standard pharmaceutical methods, including mixing the dry raw herbal components, granulation, wet screening using a sieve with 12 mesh size, drying in the oven in 400C, dry screening using a sieve with 18 mesh size, glidant to improve its flowability and lubricant to reduce friction between surfaces in mutual contact, final mixing in the Turbula Mixer for 30 minutes and the compression as a final point. Preliminary phytochemical tests and heavy metal analysis were performed according to standard methods (80).
3.3. Sample Size Determination
The minimum sample size was estimated as 200 subjects (100 cases in each group) using the Cochran formula and by considering previous similar studies.
3.4. Randomization and Masking
Referring to the http://randomizer.org URL and selecting Generate numbers item, 200 numbers in the range of 1000 to 100000 were created for the list of patients. Then, the odd and even numbers were designated as the treatment group and control group, respectively. Patients were masked to treatment allocation due to their isolated hospital rooms, but the administrators and nurses were aware of the orders.
3.5. Procedures
The prepared innovative formulation was administered orally to the patients in the treatment group 3 times a day for at least 7 days (3 tablets in each turn) and directly observed by the investigator. Also, the patients enrolled in the trial were subjected to a wide range of tests, including complete blood count (CBC) and serum AST/ALT levels in the admission state and a final stage of the discharge process. All blood samples taken from patients were sent to the hospital laboratory (Biochemistry laboratory of Imam Reza (PBUH) Hospital) for CBC and serum ALT/AST measurements. We kept in contact with the patients who were discharged from the hospital in less than 7 days to make sure that the study protocol was precisely being fulfilled (Figure 1). Patients requiring or likely to require advanced respirational support alone, or together with more organ systems, hemodynamic instability necessitating vasopressors, and oxygen saturations less than 85% were set as ICU admission criteria. Acute physiology and chronic health evaluation (APACHE) score for all patients in each arm was calculated.
3.6. Outcomes
The main outcomes included reduced length of hospitalization stay (LoS) due to improved symptoms in a lesser time, lower need for intensive care unit (ICU) admission, and decreased mortality.
3.7. Statistical Analysis
We have reported the quantitative and qualitative variables by way of mean ± standard deviation and percentage (%), respectively. The normality and non-normality of the distributions of the variables were respectively compared between the groups by means of the independent sample t-test and Mann-Whitney. Only the observed outcomes were used for data analysis. Statistical significance was based upon an alpha error probability of 5% (P < 0.05 was considered significant) and power of 80%. Variables were summarized with two-way repeated measure ANOVA with comparisons within and between-group effects. Analysis of variance was used to assess the effects of treatment interventions on the key outcomes in the current study. Data analysis was administered using SPSS version 26. The research purpose and methodology were subjected to scrutiny by the Research Ethics Committees of Tabriz University of Medical Sciences (code: IR.TBZMED.REC.1399.126). In addition, this trial is registered at the Iranian Registry of Clinical Trials (code: IRCT20200522047545N1).
3.8. Role of the Funding Source
The funder had no role in the design of the study or data interpretation. The corresponding author had final responsibility for the study and full access to all the data, as well as final responsibility for the decision to submit for publication.
4. Results
From July 26, 2020, to September 26, 2020, we screened 250 individuals, of which 200 participants with similar demographic and disease characteristics were enrolled in this randomized clinical trial. Fifty patients (i.e., 32 did not meet the inclusion/exclusion criteria, and 18 refused to participate) were excluded. All eligible patients were randomly allocated to either the treatment group (who received the standard treatment along with the compressed herbal tablets) or the control group (who only received the standard treatment) in a 1:1 ratio, i.e., 100 cases in each arm. Of whom, 200 (100%) completed the trial either in the hospitalization period for patients with LoS ≥ 7 days or after discharge from the hospital for individuals with LoS < 7 days.
Although the randomization and masking were done using the standard procedure mentioned in the Methods, due to the special conditions and pandemic emergencies, older people were in the standard. But regarding that older cases are at higher risk of death due to Covid-19, this limitation was not effective to the results of the study.
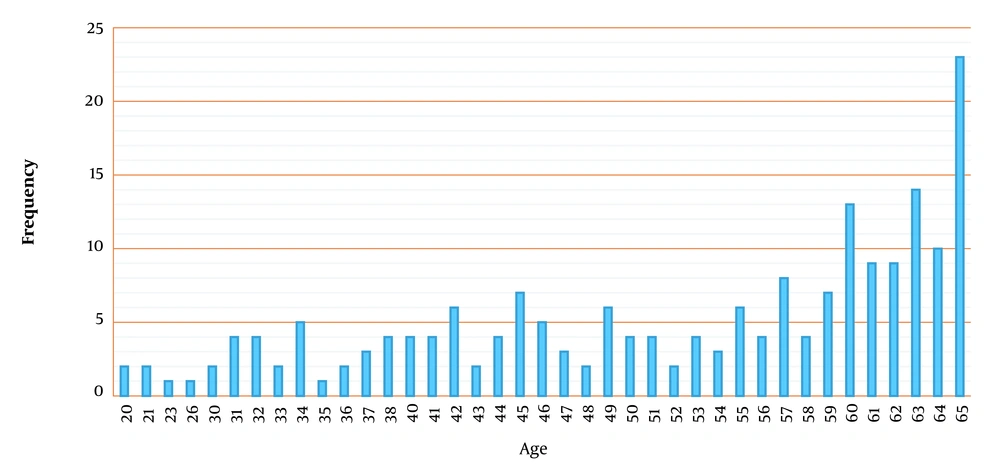
Adverse reactions incidence within 180 days after the beginning of the intervention was set as the safety endpoint. Therefore, we made a deliberate delay for 180 days in reporting the results due to monitoring patients who were enrolled in the study in the latest week of the trial procedure for possible adverse effects (Figure 2). Baseline demographic characteristics of the participants in the standard group were similar among the treatment groups in terms of gender and mean age (Table 2). The number of patients in different age groups is also reported in Figure 3. The body mass index (BMI) and APACHE score in both groups were almost equal (9.8 ± 1.2 for the standard group vs. 9.5 ± 3.6 for the treatment group).
| Variable | Frequency (%) | Number | Treatment Group | Standard Group |
|---|---|---|---|---|
| Sex | ||||
| Female | 48 | 96 | 47 | 49 |
| Male | 52 | 104 | 53 | 51 |
| Total | 100 | 200 | 100 | 100 |
| Age (range) | ||||
| 18-25 | 2.50 | 5 | 1 | 4 |
| 26-35 | 9.50 | 19 | 9 | 10 |
| 36-45 | 18 | 36 | 24 | 12 |
| 46-55 | 19.50 | 39 | 25 | 14 |
| 56-60 | 18 | 36 | 17 | 19 |
| 61-65 | 32.50 | 65 | 24 | 41 |
| Total | 100 | 200 | 100 | 100 |
Notably, patients treated within the treatment arm represented significant reductions in LoS (7.38 vs. 9.45, P = 0.030), ICU admission (6 out of 100 vs. 32 out of 100, P = 0.000), and mortality (1 vs. 19 out of 39, P = 0.000) (Table 2). The incidence of drug interactions within the study period was monitored by Prof. Varshochi and the nursing office of the hospital, and there were none to be declared. Although there were some complications of mild hyperthermia in leg toes (5 participants, without a rise in the whole-body temperature or fever status), no other minor/major adverse events were found or reported in the patients of this study.
The cases related to the above findings are reported separately in Tables 3 and 4. According to the baseline laboratory data as well the follow-up laboratory results, during the trial period, which lasted more than 180 days, there were no specific complaints from patients about drug side effects, and laboratory findings did not have any specific case of side effects and adverse effects on patients' body function. The CBC and ALT/AST values also remained unchanged. The values for CBC and ALT/AST are reported in Table 5.
| Hospitalization Days | Frequency in Treatment Group | Frequency in Standard Group |
|---|---|---|
| 2 | 1 | 0 |
| 3 | 16 | 3 |
| 4 | 4 | 27 |
| 5 | 14 | 19 |
| 6 | 13 | 14 |
| 7 | 9 | 11 |
| 8 | 7 | 3 |
| 9 | 7 | 5 |
| 10 | 3 | 2 |
| 11 | 3 | 2 |
| 12 | 1 | 2 |
| 13 | 1 | 1 |
| 14 | 1 | 2 |
| 15 | 3 | 2 |
| 16 | 3 | 2 |
| 17 | 1 | 1 |
| 18 | 0 | 1 |
| 19 | 2 | 0 |
| 20 | 2 | 1 |
| 22 | 3 | 0 |
| 23 | 1 | 0 |
| 25 | 1 | 0 |
| 28 | 1 | 0 |
| 35 | 0 | 1 |
| 37 | 1 | 1 |
| 40 | 2 | 0 |
| Treatment Group | Standard Group | Total | P Value | |
|---|---|---|---|---|
| Fate | 0.000 | |||
| Alive | 99 | 81 | 180 | |
| Dead | 1 | 19 | 20 | |
| ICU admission, number | 6 | 32 | 38 | 0.000 |
a Statistical significance was based upon an alpha error probability of 5% (P < 0.05 was considered significant) and a power for 80%. Variables were summarized with two-way repeated measure ANOVA with comparisons within and between-group effects.
| Variable | Number | Before Intervention (Mean) | After Intervention (Mean) | P Value |
|---|---|---|---|---|
| WBC (per mm3) | 200 | 6553.6 | 6789.7 | 0.98 |
| Lymphocyte (per mm3) | 195 b | 2465.1 | 2998.3 | 1 |
| LDH (U/L) | 157 b | 575.8 | 617.2 | 0.88 |
| RBC (mm3) | 200 | 5.45 | 5.42 | 0.36 |
| Hemoglobin (g/dL) | 189 b | 13.9 | 14.9 | 0.46 |
| Platelet (mm3) | 200 | 255.2 | 219.4 | 0.05 |
| AST (U/L) | 191 b | 29.9 | 32.4 | 0.57 |
| ALT (U/L) | 191 b | 21.6 | 25.1 | 0.66 |
| ESR (mm/hb) | 200 | 41.2 | 38.1 | 0.33 |
a Statistical significance was based upon an alpha error probability of 5% (P < 0.05 was considered significant) and a power for 80%. Variables were summarized with two-way repeated measure ANOVA with comparisons within and between-group effects.
b In some patients, the test was not ordered at admission or after intervention.
With the onset of COVID-19 disease in late 2019, many researchers around the world have constantly tried to find an effective treatment for the disease. However, to date, these efforts have not led to a definitive drug or treatment (8). This study was designed to evaluate the efficacy and safety of a new herbal compound formulated as compressed tablets for COVID-19 patients. The results indicated significant declines in LoS (7.38 vs. 9.45, P = 0.030), ICU admissions (6 out of 100 vs. 32 out of 100, P = 0.000), and mortality (1 vs. 19 out of 100, P = 0.000) among subjects in the intervention group.
Compressed tablets contained multi-ingredient compositions, including mainly Terminalia chebula, Glycyrrhiza glabra, Anacyclus pyrethrum, Senna alexandrina, Ferrula asafoetida, Pistacia lentiscus, Zizyphus jujuba, Crocus sativus, Echinacea angustifolia, and Hyssopus officinalis, which have been reported to have antiviral, anti-inflammatory, and immunomodulatory effects, and other additional and complementary herbal derivative and pharmaceutical excipients according to the Iranian traditional medicine and modern pharmaceutical principles to make stable and uniform tablets (Table 1). Several other groups dedicated themselves to achieving anti-SARS-Cov2 modalities worldwide, of which herbal compounds and traditional medicine have been widely developed (9-11), and their results, fortunately, played a vital role in the prevention and treatment programs developed for COVID-19 management, especially in China and South Korea, which according to the evidence, they could successfully reduce the prevalence and the mortality caused by of the disease (9, 12, 13).
In our study, we observed 21.9%, 26%, and 18% improvements in LoS (7.38 vs. 9.45, P = 0.030), ICU admission (6 out of 100 vs. 32 out of 100, P = 0.000), and mortality (1 vs. 19 out of 100, P = 0.000), respectively, in the treatment group. Individuals in the treatment group received standard treatment based on the Iranian national COVID-19 treatment protocol (remdesivir, favipiravir, hydroxychloroquine, and supportive oxygen treatment) along with the herbal compressed tablets as an intervention (Tables 3 and 4). The results of the study have been confirmed by the Committee for Ethics in Biomedical Research, Tabriz University of Medical Sciences, Iran.
5. Discussion
Given the promising results of this randomized clinical trial, the innovative formulation of herbal tablets can provide a glimmering light in the darkness of the COVID-19 pandemic to manage and overcome the disease. Reduced mortality, lower duration of hospitalization, and non-admission to the intensive care unit indicate the effectiveness of herbal compressed tablets. We believe that the special properties of the medicine (e.g., the overall host-directed regulation and certain antiviral, anti-inflammatory, strengthening, and supportive effects on patients’ immune system) will possibly be the promising mechanism of action thereof. Although due to trial limitations, potential bias, imprecision, etc. a larger study with more participants can better confirm the results of this clinical trial, verifying that the usage of the introduced tablets may plausibly facilitate the eradication of intimidating and catastrophic COVID-19 disease.