1. Background
Biosurfactants are surface-active compounds with extracellular (secondary metabolites) or cell wall-associated sources that are produced by several microorganisms such as bacteria and fungi (1-4). In contrast to synthetic surfactants that are not environmentally friendly, biosurfactants have several advantages including biodegradability, low toxicity, diversity of application, and functionality under extreme conditions (5, 6). In addition, biosurfactants are emerging as potential nanoparticle stabilizing agents and used for the treatment of wastewaters containing heavy metals (Cd++ ions) and biodegradation of model hydrocarbons and crude oil in soil (7, 8). Moreover, production of biosurfactants by microorganisms from renewable and cheaper substrates is another reason for its increased use as a green alternative to synthetic surfactants.
Several reports indicated that bacterial species such as Acinetobacter (9), Pseudomonas (10, 11), Bacillus (12), Lactococcus (13), and Nocardia mediterranei (14) are applicable biosurfactants sources. Much research has shown that bacterial biosurfactants possess antimicrobial properties (9, 10, 15, 16). Mostafapour et al. have shown that a biosurfactant produced by Acinetobacter species has anti-Gram positive and negative bacteria effects in vitro (9). In addition, Gomaa examined the biological properties of biosurfactant produced by B. licheniformis and found that it has great potential for the biotechnological and biopharmaceutical applications (17). Moreover, a synergistic effect was found against plant pathogenic fungi and pathogenic bacteria when silver nanoparticles were used with biosurfactants (18). Furthermore, Basit et al. recommended lipopeptide biosurfactant produced by B. cereus as a safe antimicrobial and antioxidant agent (19).
Producing biosurfactants by fungi is limited to some species of Candida, Pseudozyma, Yarrowia, Penicillium and Aspergillus species (5, 20-23). Recently, the production of biosurfactants from Rhodotorula glutinis (24), R. mucilaginosa (25), and R. paludigena (26) has been investigated by researchers. It seems that Rhodotorula species are the main producers of biosurfactants; hence, they have new possibilities for industrial application. The antibacterial, antifungal, and antiviral activities of several biosurfactants have been reported by researchers and used for new antibiotic therapy (10, 12, 17, 20).
Although many types of biosurfactants including, glycolipids, rhamnolipids, sophorolipids, mannosylerythritol lipids, phospholipids, polymeric compounds, mycolic acids, and lipopolysaccharides are distinguished (27), it seems that lipopeptides represent remarkable biological activities, such as antibacterial, antifungal, antitumor, antiviral, and antiadhesive activities (15, 17). Environmental applications of biosurfactants were focused by many researchers and during the last decades, the biomedical field applications have been carried out. New more-active antifungal agents with fewer side effects are usually demanded by researchers, clinicians, and patients.
2. Objectives
In the present study, we evaluated the biosurfactant production ability of different strains of Rhodotorula species in laboratory conditions. Furthermore, the antifungal activity of produced biosurfactant was assessed against several fungi (molds and yeasts).
3. Methods
3.1. Organisms
In the present study, 54 strains of Rhodotorula species including, R. glutinis (48 strains), R. mucilaginosa (two strains), R. minuta (two strains), and Rhodotorula species (two strains) were examined for biosurfactant production. All strains had been already collected from different sources, identified, and kept in distilled water in the Department of Medical Mycology affiliated to Ahvaz Jundishapur University of Medical Sciences, Iran (28). All the tested strains were cultured on Sabouraud dextrose agar (SDA, Merck, Germany) slants and incubated at room temperature for four to five days for strains recovery.
3.2. Screening for Biosurfactant Production
Each of the 54 strains of four species of Rhodotorula was separately inoculated into 5 mL portions of Sabouraud dextrose broth (SDB, Merck, Germany) in test tubes and incubated at ambient temperature in a shaker incubator for four to six days. Then, the cultures were centrifuged at 3000 g for 10 minutes to obtain the cell-free broth supernatant containing biosurfactant. Biosurfactant production was confirmed by various standard methods including oil displacement (21), drop collapse (29), and haemolysin tests (30).
3.3. Biosurfactant Production on a Laboratory Scale
At this stage, only was one strain of R. glutinis selected with high ability to produce biosurfactant. The isolate was inoculated into several Erlenmeyer flasks (250 mL volume) containing 100 mL of SDB and incubated in the shaking incubator at 29ºC for one week. The free cell supernatant was removed using centrifugation at 3000 g for 10 minutes. The same volume of supernatant (crude biosurfactant) and chloroform-methanol (2:1 v/v) were mixed, and then the biosurfactant was extracted by centrifugation (26, 31). The gather biosurfactant was dried and kept at -20ºC until use.
3.4. Antifungal Assay
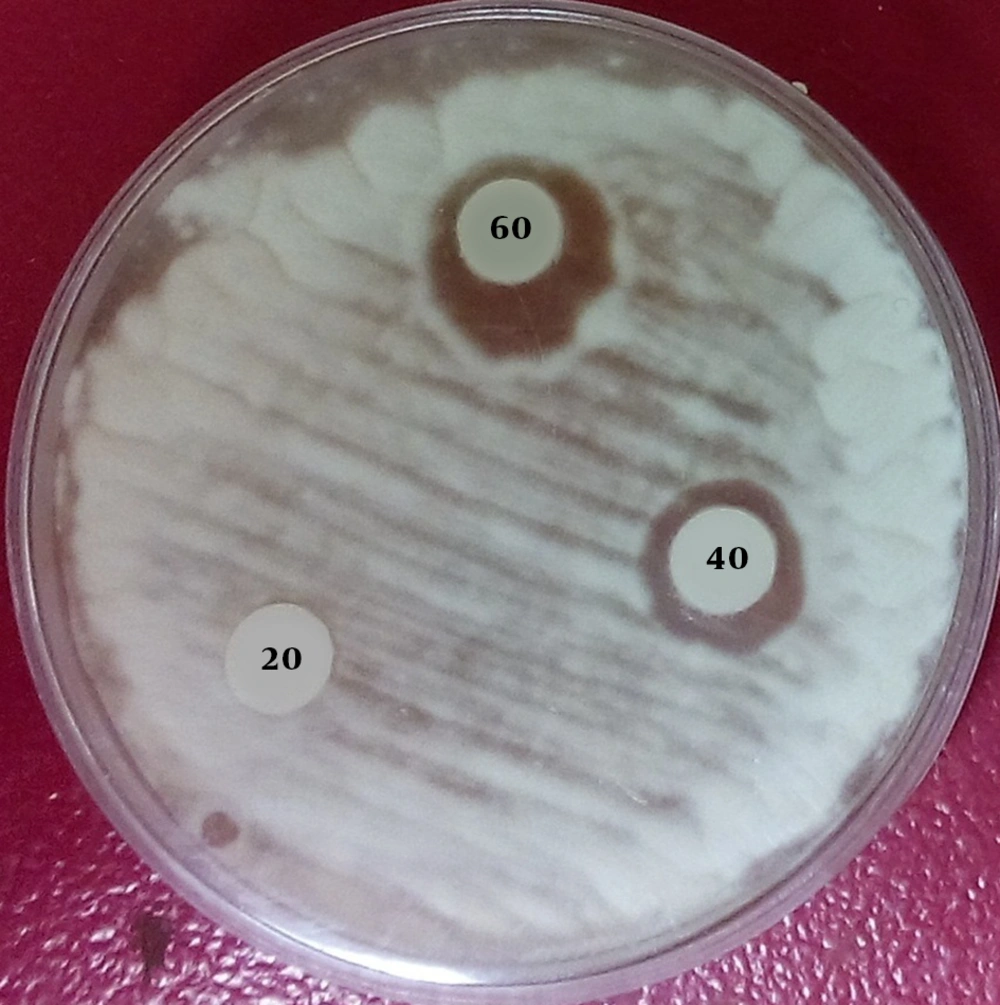
The antifungal activity of the biosurfactant was examined against different strains of Candida albicans (seven isolates), R. glutinis (two isolates), Aspergillus niger (one isolate), Alternaria sp. (one isolate), Rhizopus sp. (one isolate), and Syncephalastrum sp. (one isolate). The antifungal activity was evaluated by the disk diffusion method. Briefly, a standard suspension of each strain was prepared in sterile distilled water and then, 10 µL was spread on the surface of SDA plates in duplicate. Subsequently, 50 mg of the crude biosurfactant was dissolved in 1 mL of DMSO/ethanol completely. Finally, three blank disks were put on each plate and an aliquot of diluted biosurfactant (20, 40, 60, 80, 100, and 120 µL) was added into each disk. The plates were incubated at 29 and 35ºC for molds and yeasts, respectively. The hyaline haloes (without fungal growth) around the disks were measured and the minimum inhibitory concentration (MIC) for each strain was calculated (Figure 1).
4. Results and Discussion
In the present study, although all the tested strains were capable of producing biosurfactant in vitro, the degree of biosurfactant was different among the strains. Out of 54 Rhodotorula strains, only could four strains of R. glutinis comparatively show higher zones in the oil displacement test (2.1 - 2.5 cm) confirmed by drop collapse and haemolysin tests (Table 1). These results indicated that they had a high potential for biosurfactant production. Mahalingam and Sampath believe that the oil displacement technique is very sensitive for detecting biosurfactants even at low levels (29). In this test, a larger diameter represents a higher surface activity of the biosurfactant (32). Rhodotorula species are new sources for producing different biosurfactants. Extracellular glycoprotein biosurfactants from R. glutinis (24) and astaxanthin from R. mucilaginosa (25) are two types of biosurfactants that have been recently detected. Previous studies have shown that the biosurfactants produced by R. glutinis are composed of lipids (5).
| Halo Diameter (cm) | Rhodotorula | ||||
|---|---|---|---|---|---|
| R. glutinis | R. minuta | R. mucilaginosa | Rhodotorula Species | Total | |
| > 0.5 (+1) | 11 (20.4%) | 0.0 | 0.0 | 0.0 | 11 (20.4%) |
| 0.6 - 1 (+2) | 10 (18.5%) | 2 (3.7%) | 1 (1.8%) | 1 (1.8%) | 14 (25.8%) |
| 1.1 - 1.5 (+3) | 14 (25.9%) | 0.0 | 1 (1.8%) | 1 (1.8%) | 16 (29.5%) |
| 1.6 - 2 (+4) | 9 (16.7%) | 0.0 | 0.0 | 0.0 | 9 (16.7%) |
| 2.1 - 2.5 (+5) | 4 (7.4%) | 0.0 | 0.0 | 0.0 | 4 (7.4%) |
| Total | 48 (88.9%) | 2 (3.7%) | 2 (3.6%) | 2 (3.6%) | 54 (100%) |
Due to developing fungal resistance to antifungal agents, a lot of attention has been paid to new natural compounds with antifungal properties. In the present study, we found that the growth of all tested fungi including yeasts and molds strains completely was inhibited by 40 µL of the biosurfactant. Despite their potential for biomedical fields, only have a few studies been carried out on the antifungal activity of biosurfactants. Antifungal activities of biosurfactants produced by Lactobacillus lactis (13), B. subtilis (12), and P. aeruginosa (10) were investigated by several researchers. Furthermore, Ceresa et al. have shown that the biosurfactant produced by L. brevis has antibiofilm formation activity in C. albicans (33). A synergistic effect of surfactin with ketoconazole against C. albicans was also considered by Liu et al. (27). Furthermore, Halvaeezadeh and Zarei Mahmoudabadi showed that the produced biosurfactant by R. paludigena in combination with caspofungin have synergistic effects against C. albicans strains (26).
Anti-adhesive activity, permeabilizing ability, and cellular damaging ability are reported as the possible effective mechanisms of biosurfactants (13, 16, 20). Furthermore, biosurfactants could inhibit the adhesion of Candida to the silicon surface (33).
4.1. Conclusions
In conclusion, Rhodotorula species are applicable organisms for the production of biosurfactants and R. glutinis strains have the greatest ability to produce biosurfactants among other species. Furthermore, our results demonstrated that the biosurfactant produced by R. glutinis had a valuable potential for biopharmaceutical applications.