1. Background
Methotrexate (MTX), is an antagonist of folic acid, and a key drug for the treatment of different types of cancer (1). Also, MTX is used in many other diseases like different types of arthritis, some kinds of leukemia, and inflammatory bowel disease (Crohn's disease and ulcerative colitis) (2). However, MTX side-effects limit its application, with hepatic complications (hepatitis, cirrhosis, hepatic fibrosis, and hepatocellular necrosis) being the most severe (1).
The mechanism responsible for MTX hepatotoxicity is not clearly defined. Some researchers mention that it is associated with oxidative stress and enhanced inflammatory processes (3). They proposed that MTX results in lipid peroxidation and also lowers the levels of antioxidant enzymes like catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione reductase (GR), indicating oxidative stress and disturbing the antioxidant enzyme defense systems (1, 4). There are documents that prove in the processes of hepatotoxicity induced by MTX, malondialdehyde (MDA, an indicator of enhanced lipid peroxidation) increases (3, 5) and reduced glutathione (GSH, a defender in the redox system) decreases (4). Another possible mechanism for hepatic injury by MTX is that MTX can increase the tissue levels of NO (6), and NO can consequently induce cell apoptosis (7). There are also studies that confirm the role of increased production of pro-inflammatory cytokines in the pathogenesis of liver injury in MTX-treated rats (3, 5, 6). Besides, MTX hepatotoxicity, like any kind of hepatic injury, can increase hepatic enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (3, 5, 8).
Ginger (Zingiber officinale Roscoe, family: Zingiberaceae) is a plant that produces one of the most common spices used all over the world. It has a pungent odor and taste and adds a special flavor to edibles (9). Zingerone is present in a significant amount in Ginger and is known to have potent pharmacological activities (10). Ginger is a traditional remedy, used for the treatment of numerous diseases for years (11). It has a powerful effect on scavenging ROS, free radicals, peroxides, and various other damaging oxidants (12-15). Also, it shows anti-inflammatory (16) and antidiareal properties (17, 18), reduces radiation-induced stress (19, 20), and exerts anticancer (21-23), antiemetic (24-26), and lipolytic effects (27, 28).
2. Objectives
In this study, we decided to use an experimental rat model to evaluate if the most important constituent of ginger, zingerone, can reduce the severity of the dangerous side effects of methotrexate, i.e., hepatotoxicity, as one of the commonly used prescribed drugs. Regarding the increasing popularity of natural products for use as drugs, if it works, ginger can be a potential natural treatment for liver injury in patients who take MTX on a chronic basis.
3. Methods
3.1. Chemicals
All the chemicals were obtained from Sigma (St Louis, MO, USA), including zingerone, MTX, 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), thiobarbituric acid (TBA), Bradford reagent, trichloroacetic acid (TCA), GSH, and bovine serum albumin (BSA).
3.2. Animals and Study Design
The Central Animal House of Ahvaz Jundishapur University of Medical Sciences supplied 37 adult male Wistar rats weighing 180 - 220 grams. They were kept in standard rat cages under the room temperature of 24 - 26°C and a 12-h light/dark cycle and had free access to standard rat food and water. We divided them into five groups of seven animals. Before starting the treatment, they were left for seven days to acclimatize to the new environment. The groups included:
1. Control group: Receiving normal saline (N/S), once daily, by gavage, for 10 days, and N/S intraperitoneally (i.p.), a single dose on the ninth day.
2. Methotrexate group (MTX): Receiving normal saline, once daily, by gavage, for 10 days, and MTX intraperitoneally (i.p.), a single dose (20 mg/kg) on the ninth day.
3. Zingerone 25 (ZG25): Receiving zingerone suspension in N/S (25 mg/kg), once daily, by gavage, for 10 days, and MTX intraperitoneally (i.p.), a single dose (20 mg/kg) on the ninth day,
4. Zingerone 50 (ZG50): Receiving zingerone suspension in N/S (50 mg/kg), once daily, by gavage, for 10 days, and MTX intraperitoneally (i.p.), a single dose (20 mg/kg), on the ninth day.
5. Zingerone 100 (ZG100): Receiving zingerone suspension in N/S (100 mg/kg), once daily, by gavage, for 10 days, and MTX intraperitoneally (i.p.), a single dose (20 mg/kg) on the ninth day.
The protocols used in this study were approved by the Animal Care and Use Committee of Ahvaz Jundishapur University of Medical Sciences (Ethics code: IR.AJUMS.ABHC.REC.1398.034). The authors confirm that no changes were made to the initial protocols.
3.3. Sample Collection and Preparation
After 24 hours of the last administration, rats received a dose of ketamine and xylazine (60/6 mg/kg, i.p.) for the induction of anesthesia, and 2 cc of whole blood was taken from the jugular vein and centrifuged for 15 minutes at 3,000 rpm. The serum was isolated and stored at -20°C until further analysis for ALT, AST, and ALP. Then, liver tissues were harvested. Some of the liver tissue, which was needed for histological studies, was fixed in phosphate-buffered formalin (10%). Another part of the liver tissue underwent homogenization with the chilled Tris–HCl buffer (0.1 M, pH 7.4) and the supernatant was kept at -80°C for assessing tissue protein concentrations by the Bradford method (29) using crystalline bovine serum albumin as standard, as well as for all tissue biochemical assays.
3.4. Serum Biochemical Assays
The activities of serum ALP, ALT, and AST were measured by colorimetric kits (Wiesbaden, Germany) using a spectrophotometer (UNICO Instruments C., Model 1200, USA).
3.5. Tissue Biochemical Assays
3.5.1. Malondialdehyde (MDA)
The MDA level was measured based on Aust’s method (30). The tissue supernatant (0.5 mL) was added into TCA (1.5 mL; 10%, w/v)-containing tubes. Afterward, tubes were centrifuged for 10 minutes at 3,400 rpm. Then, 1.5 mL of the supernatant was added to the tubes with TBA (2 ml; 0.67% w/v). The mixture was kept in boiling water for 30 minutes, and a pink solution was formed; then, the mixture was cooled down immediately. To measure absorbance at 532 nm, a spectrophotometer (UV-1650 PC; Shimadzu, Japan) was used, and the level of MDA (nmol/mg protein) was determined using a specific absorbance coefficient related to TBA-MDA.
3.5.2. Reduced Glutathione (GSH)
We assessed GSH based on the reaction with Ellman’s reagent (DTNB) (31). For precipitating the tissue homogenate supernatant, 100 mL of TCA (25%) was used. Then, by using a centrifuge, the precipitate was removed, and 0.1 mL of the supernatant was blended with 2 mL of DTNB (0.5 mM). Absorbance at 412 nm was measured by a UV–Vis spectrophotometer (Shimadzu, Japan). Final results extrapolated the GSH content from the standard curve (nmol/mg protein).
3.5.3. Protein Carbonyl (PC)
In the assay, 500 mL of DNPH (0.1%) in 2N HCl was mixed with 500 mL of the supernatant and kept in dark for one hour. The product was mixed with TCA (20%) and then centrifuged. The supernatant was thrown away. The pellets were washed out with 0.5 mL of ethanol–ethyl acetate and were suspended in Tris-buffered 8M guanidine HCl (1000 mL). A UV–Vis spectrophotometer (UV-1650 PC, Shimadzu, Japan) was used to measure absorbance at 370 nm (nmol of carbonyl/mg protein) (32).
3.5.4. Superoxide Dismutase (SOD), Nitric Oxide (NO), Glutathione Peroxidase (GPx), and Catalase (CAT)
The level of NO (nmol/mg protein) and the activities of SOD, GPx, and CAT enzymes (units/mg of protein) in the liver were measured by specific kits (ZellBio, Germany), following the manufacturer’s instructions.
3.5.5. Tumor Necrosis Factor-α (TNF-a) and Interleukin 1β (IL-1β)
Rat TNF-α and IL-1β ELISA kits (IBL, USA) were used following the manufacturer’s instructions for measuring TNF-α and IL-1β tissue levels (pg/mg protein).
3.6. Histopathological Examination
After at least 24 hours, the samples were pulled out from 10% formalin, which was used for fixing the tissue. The dehydration of liver tissue was accomplished by embedding in paraffin and a sequence of ethanol solutions. The tissues were cut into 5 μm sections and stained with Hematoxylin & Eosin (H&E) stain. A light microscope (OLYMPUS, Japan) was used for observing the slides blindly. The congestion of RBC, inflammatory cell infiltration, fat deposit, and pyknosis were assessed as inflammation indices. Semi-quantitative analysis of these indices was accomplished based on the following scoring system: (1) 0 = normal tissue; (2) 1 = mild hepatic injury; (3) 2 = moderate hepatic injury; and (4) 3 = severe hepatic injury. The total score was calculated by obtaining the average of all scores in each group.
3.7. Statistical Analysis
The Graph Pad Prism 5.0 (San Diego, CA) software was used for data analysis. We used one-way ANOVA and Tukey’s post hoc tests for multiple comparisons. A P value of < 0.05 was considered significant, and the results were reported as means ± SD.
4. Results
4.1. Zingerone Effect on Serum ALP, AST, and ALT
Serum ALP, AST, and ALT were significantly higher in MTX-intoxicated rats than in the control group (P < 0.05). The administration of zingerone (50 and 100 mg/kg) significantly lowered the serum levels of ALT and AST in rats with hepatotoxicity (P < 0.05). Besides, ALP was lowered significantly only by zingerone (100 mg/kg, P < 0.05) (Figure 1).
4.2. Zingerone Effect on MDA, NO, and PC in Liver Tissue
The liver tissue levels of MDA, NO, and PC were significantly higher in rats intoxicated with MTX than in the control group (P < 0.05). The MDA tissue levels were lowered significantly by the administration of zingerone (50 and 100 mg/kg, P < 0.05). Besides, NO tissue levels could significantly be lowered only by zingerone (100 mg/kg, P < 0.05). But, PC tissue levels could be lowered by all doses of zingerone (P < 0.05) (Figure 2).
4.3. Zingerone Effect on Activity of SOD and CAT Enzymes in Liver Tissue
The SOD and CAT enzymes had significantly lower activity in MTX-intoxicated rats than in the control group (P < 0.05). The SOD activity was accelerated significantly only by the administration of zingerone 100 mg/kg (P < 0.05). But, both doses of 50 and 100 mg/kg of zingerone could significantly increase the activity of CAT (P < 0.05) (Figure 3).
4.4. Zingerone Effect on GSH Content and Activity of GPX Enzyme in Liver Tissue
The content of GSH and the activity of the GPX enzyme were significantly lower in rats intoxicated by MTX than in the control group (P < 0.05). The GSH content was accelerated significantly by the administration of zingerone 50 and 100 mg/kg (P < 0.05). But, all doses of zingerone could significantly increase the activity of GPX (P < 0.05) (Figure 4).
4.5. Zingerone Effect on TNF-α and IL-1β in Liver Tissue
Liver tissue levels of TNF-α and IL-1β were significantly higher in rats intoxicated by MTX than in the control group (P < 0.05). The TNF-α tissue levels were lowered significantly by the administration of zingerone (50 and 100 mg/kg, P < 0.05). But, IL-1β tissue levels could be lowered by all doses of zingerone (P < 0.05) (Figure 5).
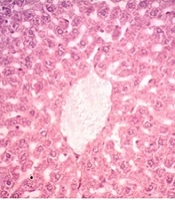
4.6. Zingerone Effect on Histopathological Features of Liver Tissue
The histopathological exam of liver tissue in the control group revealed a normal structure of cells, an intact cytoplasm, a well-defined nucleus, portal tracts, and central veins (Figure 6A and Table 1). The congestion of RBCs (C), infiltration of inflammatory cells, severe cellular damage, fat deposition, and nuclear pyknosis (P) were seen in the group treated with MTX (Figure 6B and Table 1). Zingerone with all doses significantly decreased the above-mentioned distractive changes, when compared with the MTX group (Figure 6C - E, respectively, and Table 1), except for one feature (congestion of RBC in the ZG25 group).
| Histological Criteria | Groups | ||||
|---|---|---|---|---|---|
| Control | MTX | ZG25 | ZG50 | ZG100 | |
| Congestion of RBC | 0.3 ± 0.1 | 2.6 ± 0.2 a | 2.3 ± 0.1 a | 1.3 ± 0.3 a, b | 0.5 ± 0.3 b |
| Infiltration of inflammatory cells | 0.4 ± 0.2 | 2.5 ± 0.3 a | 2.1 ± 0.1 a, b | 1.0 ± 0.2 a, b | 0.8 ± 0.1 a, b |
| Fat deposit (%) | 3.8 ± 0.5 | 28.3 ± 3.7 a | 25.1 ± 2.1 a, b | 11.9 ± 1.1 a, b | 6.9 ± 0.8 b |
| Pyknosis (%) | 1.8 ± 0.2 | 30.5 ± 4.5 a | 23.3 ± 3.2 a, b | 16.1 ± 2.1 a, b | 4.1 ± 1.6 b |
a Significant difference with the control group, P < 0.05.
b Significant difference with the MTX group, P < 0.05.
5. Discussion
Methotrexate (MTX) is one of the medicines on the World Health Organization's list of essential medicines. The MTX chronic administration even at very low doses is toxic to the liver, kidney, respiratory, reproductive, and hematopoietic systems. The liver is the organ, which is affected most by this drug (1). Using natural products for treating diseases is now increasing due to lifestyle changes. People all around the world have become more alert about health and nutrition and prefer to take natural products instead of synthetic drugs, due to their fewer side effects (11). In this study, we evaluated the role of zingerone (one of the main constituents of the natural substance, ginger) on MTX-induced liver injury in a rat model, from diverse points of view, including redox system, inflammation, and liver enzymes. Finally, we did histopathological examinations to see if they confirm our results.
Injury to the liver (acute or chronic) results in an increment in serum concentrations of liver aminotransferases (AST and ALT). Besides, Alkaline Phosphatase (ALP) levels can mostly be elevated by liver and bone diseases (33). The present study showed that the administration of zingerone could decrease ALT, AST, and ALP by at least one dose of zingerone significantly. Amin et al. and Mir et al. confirm our study by showing the protective effect of zingerone on lead- (8) and cyclophosphamide (34)-induced hepatoxicity, respectively, regarding liver function markers.
The imbalance between reactive oxygen and nitrogen species (RONS) production and antioxidant defense systems can result in oxidative stress. Besides, RONS is the result of many endogenous and exogenous processes (35). Enzymes such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) are the main cellular antioxidant defensive systems that can protect cells against ROS-induced damage (36).
Lipids are the most important targets of oxidative stress. Lipid oxidation gives rise to the production of malondialdehyde (MDA), which has potentially mutagenic effects (37). This study demonstrated that zingerone (50 and 100 mg) could decrease the main product of lipid peroxidation (MDA), showing its ability to defeat oxidative damage in MTX-induced hepatotoxicity. Mir et al. also showed that zingerone could attenuate MDA elevation induced by cyclophosphamide-induced liver injury, which confirms our results (34).
Although nitric oxide (NO) plays an important role in several vital physiological functions (38), it is also a poisonous, highly reactive, short-lived, and unstable free radical gas (39), which can induce apoptotic cell death in neuronal cells (7, 34). When NO reacts with superoxide radicals, it yields peroxynitrite, which can cause membrane peroxidation, DNA damage, and GSH content depletion (34). The current study showed that zingerone (100 mg/kg) could significantly decrease the level of liver tissue NO in MTX-induced hepatic injury. Wei et al. declared that ROS is probably involved in a series of events that result in cell injury by NO (7). Therefore, we can conclude that zingerone shows a protective effect against cell apoptosis and possibly performs this protection via decreasing ROS in the liver. Mir et al. also showed a result parallel to our results; they showed the protective effects of zingerone regarding reduced nitrite production in cyclophosphamide-induced hepatic injury (34).
Moreover, RONS can oxidize proteins and give rise to the production of reactive molecules known as protein carbonyl (PC). A reliable indicator of protein oxidation is the quantity of PC in blood and tissues (40). This study showed that zingerone with all doses used could decrease the elevated tissue levels of PC in the liver of MTX-treated rats. In another study, Hosseinzadeh et al. showed that zingerone could decrease the content of PC in renal tissue in gentamicin-induced nephrotoxicity in rats (41). As protein carbonyl is an indicator of the existence of free radicals (42), it can be concluded that zingerone has an antioxidant property.
Superoxide dismutase (SOD) is an important antioxidant defense system in nearly all living cells exposed to oxygen (43). Besides, SOD can halt the damaging potential of the superoxide onion. Also, SOD catalyzes the dismutation of superoxide to oxygen plus hydrogen peroxide (H2O2) (44). Hydrogen peroxide is also detrimental and can be degraded by catalase (43). In the current study, we proved that zingerone (100 mg/kg) could increase the activity of both SOD and CAT in the liver of MTX-affected rates. Amin et al. and Mir et al. confirmed our results about the increasing activity of CAT and SOD by using zingerone (8, 34).
Reduced glutathione (GSH) is at the center of one of the most important antioxidant systems. It is capable of scavenging RONS and acting as a cofactor for an antioxidant enzyme, glutathione peroxidases (GPX) (45). In this study, we proved that these two important agents in the redox system could be increased by using zingerone (50 and 100 mg/kg) in rats with MTX-induced hepatic injury. Mir et al. showed results parallel to our study results regarding the increments of both GPX activity and GSH liver content by zingerone (34). Amin et al.’s study, parallel with our study, showed the increased activity of GPX in hepatic injury by administering zingerone (8).
This study showed that zingerone could decrease pro-inflammatory cytokines (IL-1β and TNF-α). As known, ROS production leads to neutrophil infiltration and proinflammatory cytokine release, and this can result in apoptosis, cell damage, and death (6). Thus, it is concluded that zingerone can protect hepatic injury via decreasing inflammation and ROS features. Kucukler et al. also showed similar results; they declared that zingerone could decrease these cytokines that are responsible for inflammation and apoptosis in vancomycin-induced hepatoxicity (46).
The current study showed a great improvement in MTX-induced histopathological changes in liver tissue after zingerone administration. Zingerone, at least at two doses, could reduce all features assessed for hepatic cell damage. Amin et al. (8) also stated a significant positive change in hepatic and renal histopathology by zingerone, which confirms this study.
5.1. Conclusion
Zingerone can improve hepatic injury induced by methotrexate in rats regarding the redox system features, inflammation, and histological changes. This can make humans hopeful for using Ginger in the future for attenuating the hepatic side effects of MTX when used chronically.
![Effect of zingerone on serum enzymes of ALT, AST, and ALP in rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX: methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)]. Effect of zingerone on serum enzymes of ALT, AST, and ALP in rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX: methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/27a7d9ab8af8ff8e40eb666820477a78b25cdb0e/jjnpp-118745-i001-F1-preview.webp)
![Effects of zingerone on liver tissue levels of MDA, NO, and PC in rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)]. Effects of zingerone on liver tissue levels of MDA, NO, and PC in rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/7c6b7126e73f0fc9144902a35d091ce2e6cec742/jjnpp-118745-i002-F2-preview.webp)
![Effects of zingerone on the activity of SOD and CAT enzymes in liver tissue of rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)]. Effects of zingerone on the activity of SOD and CAT enzymes in liver tissue of rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/4c434c39012df02bd68cd39b55c18e0c5eee4cac/jjnpp-118745-i003-F3-preview.webp)
![Effects of zingerone on the content of GSH and activity of GPX enzyme in liver tissue of rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)]. Effects of zingerone on the content of GSH and activity of GPX enzyme in liver tissue of rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/6dccec0009401323266dc9645e883fa72baba3cf/jjnpp-118745-i004-F4-preview.webp)
![Effects of zingerone on liver tissue levels of TNF-α and IL-1β in rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)]. Effects of zingerone on liver tissue levels of TNF-α and IL-1β in rats treated with MTX (mean ± SD; n = 7) [ZG, zingerone; MTX, methotrexate; * Significant difference with the control group (P < 0.05); # Significant difference with the MTX group (P < 0.05)].](https://services.brieflands.com/cdn/serve/3170b/400610c62847e03b746a0d0fb3f6f6910957dbb2/jjnpp-118745-i005-F5-preview.webp)
![Histopathological observations showing the effects of zingerone on rat liver tissue. (sections stained with Hematoxylin & Eosin, magnification X 200) [C, congestion of RBC; P, pyknosis; A, control; B, MTX; C, ZG25; D, ZG50; E, ZG100]. Histopathological observations showing the effects of zingerone on rat liver tissue. (sections stained with Hematoxylin & Eosin, magnification X 200) [C, congestion of RBC; P, pyknosis; A, control; B, MTX; C, ZG25; D, ZG50; E, ZG100].](https://services.brieflands.com/cdn/serve/3170b/3b42eb31afba0726a68f29e156a0f5de051c47cc/jjnpp-118745-g001-F6-preview.webp)