1. Background
The term cancer refers to genetic changes (such as mutations) disrupting the normal division of cells and cell differentiation (1). It is estimated that by 2060, cancer will be the first and most important reason of human death (2). Breast cancer (BC) is the main malignancy diagnosed in women and is the second leading reason of global death worldwide after lung cancer (3). Asian countries have the highest prevalence of BC (4). In Iran, the prevalence of this type of cancer accounts for 23% of all cancers (5). The incidence of this type of cancer increases with aging, and it often occurs in women aged 40 years or older (6).
Current methods of BC therapy include surgery, chemotherapy, radiotherapy, hormone therapy, and immune-therapy. However, these methods have their own limitations. Chemical drugs on the market are less popular due to their high side effects. Therefore, anti-cancer agents with natural sources have been considered recently (7). Herbal products can specifically stop the growth of cancer cells and show less toxicity to healthy cells, and are a good strategy for treating all types of cancer (8). Among the well-known medicinal plants, there are genera of the Umbrella family called Eryngium, which have antioxidant (9-12), anti-cancer (13, 14), anti-bacterial and anti-fungal (15, 16), anti-inflammatory (17), analgesic (18), anti-diabetic (19), anti-seizure (20) and other effects. The cytotoxicity of some Eryngium species has been mentioned in previous literature.
2. Objectives
Considering the natural growth of E. thyrsoideum in Iran and the high prevalence of BC, the current research aimed to investigate the anti-cancer effects of E. thyrsoideum on BC cell lines.
3. Methods
3.1. Preparation of Plant Samples
Eryngium thyrsoideum plant was gathered in October 2019 from Maragheh region in East Azerbaijan province, Iran. The plant was distinguished by a doctor in the herbarium of Tabriz University of Medical Sciences (NO: TBZ-FPH 1250). First, the aerial parts of E. thyrsoideum were dried in open air and then turned into a very fine powder by electric mill. The obtained powder was very light and had a low density.
3.2. Preparation of Medicinal Plant Powder and Extraction
The shoots of E. thyrsoideum were kept in the open air conditions and ground by electric mill to obtain a dried and fine powder. Next, 200 g of the powder was extracted by Soxhlet method with solvents of n-Hexane (n-Hex), methanol (MeOH), and dichloromethane (DCM). Then, the extract was dried at 45°C under low air pressure using a rotary evaporator (21). After making sure the extracts were completely dry, they were dissolved in dimethyl sulfoxide (DMSO) solvent. The amount of DMSO was less than 0.1%, which did not have a significant cytotoxic effect (22). DMSO control was also included in the tests, and cell viability was determined according to DMSO control.
3.3. Fractionation of Methanolic Extract by Solid Phase Extraction Method
Solid phase extraction (SPE) method was used to prepare different fractions of MeOH extract. For this aim, 2 g of dried MeOH extract was completely dissolved in a minimum amount of water and methanol mixture with a ratio of 10:90. The solution was transferred to the Sep-pack C18 cartridge and washed with 200 mL of solvent (water-methanol mixtures in ratios of 90:10, 80:20, 60:40, 40:60, 20:80, and 0:100, respectively). Final result was the formation of six fractions, all of which were completely dried and weighed using a rotary evaporator at 45°C under a vacuum. The whole extract and its fractions were stored in the freezer until utilization.
3.4. Chemicals
All solvents used in this study were provided by Dr. Mojallali Industrial Chemical Complex Co. (Tehran, Iran). All chemicals were analytical grade.
3.5. Culture Method and Cell Lines
BC cell lines (MCF-7 and MDA-MB-231), as cancerous cells, and human foreskin fibroblasts (HFF-2) were bought from the National Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). Cell lines were incubated in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1% Penicillin-Streptomycin antibiotics in sterile flasks at 37°C, 5% CO2, and 95% humidity (23). Among BC cell lines, MDA-MB-231 is more sensitive than MCF-7 cell line (24). MDA-MB-231 cell line generally accounts for 15 - 20% of all BC cases and is categorized as a basal type of BC cell line with triple-negative for ER (estrogen), PR (progesterone), and HER2 (human epidermal growth factor receptor 2), while MCF7 cell line is easily treated and is known as luminal type of BC cell line with ER and PR positive (25, 26). In addition, the MDA-MB-231 cell line has an elongated morphology and grows rapidly, but MCF-7 grows in clusters and is slow-growing (27).
3.6. Preparation of Plant Samples
To prepare the stock for each extract, 10 mg of each dried each dried powder was dissolved in sterile microtubes at a minimum concentration of DMSO. The final volume of each microtube was 1 mL using complete culture medium. To prepare the stocks, the desired concentrations of 10, 20, 50, 100, 150, 200, and 300 μg/mL were prepared. Also, the corresponding stock was prepared from each fraction. Concentrations of 50, 100, 150, 100, and 250 μg/mL were prepared from the resulting stocks. In the prepared concentrations, the amount of DMSO was less than 0.1%, which did not have significant cytotoxic effect. DMSO control was also included in the tests, and cell viability was determined according to DMSO control. Finally, the samples were sterilized using a Millipore syringe filter and stored in the refrigerator until use.
3.7. In Vitro Cytotoxicity Assay
MTT assay was used to determine the toxicity of substances on cell lines by enzyme-linked immunosorbent assay (ELISA) plate reader (Bio tech, Bad Friedrichshall, Germany) at 570 nm according to the methods described by Montazersaheb et al. (28). Then, the half-maximal inhibitory concentration (IC50) cell suspension with 5 × 103 cells/mL was inoculated in 96-well microplates for 24 hours (29, 30). The negative control test was DMSO, and positive control test was the cells without extract.
3.8. Evaluation of Apoptosis by Flow Cytometry Technique
To perform this test, 2 × 105 cells were inoculated in 6-well plates. Flow cytometry technique was carried out to evaluate the apoptosis rate according to the method described by Fathi et al. (29) at 488 nm (excitation wavelength) and 617 nm (emission wavelength). The flow cytometric data were analyzed by Cell quest pro software (IBM, CA, USA) (31, 32).
3.9. Statistical Analysis
The Results of UV absorption at 570 nm were evaluated using GraphPad Prism 8.0.1 software, and nonlinear regression analysis was used to obtain IC50. The IC50 value of the samples indicates the concentration of the sample, which inhibits 50% of cell growth. The evaluation of the P-value was calculated using the analysis of variance (ANOVA) and Tukey’s post hoc test.
4. Results
4.1. Amounts of Extracts and Fractions
The aerial parts of E. thyrsoideum (100 g) were extracted by Soxhlet apparatus using n-Hex, DCM, and MeOH solvents and reported as 3.42 g, 2.14 g, and 6.15 g, respectively (Table 1). The results of MeOH extract’s fractions as the strongest extract are also shown in Table 1.
| Weight of Samples | n-Hex | DCM | MeOH | |||
|---|---|---|---|---|---|---|
| plant extract (g/100 g of plant) | 3.42 | 2.14 | 6.15 | |||
| Fractions (%) | 10 | 20 | 40 | 60 | 80 | 100 |
| MeOH extract fraction (g/100 g of extract) | 5.8 | 3.5 | 5.5 | 4.2 | 4.9 | 6.2 |
Results of Weighing n-Hex, MeOH, and DCM Extracts and MeOH Extract’s Fractions from 100 g of Eryngium thyrsoideum Powder
4.2. Cytotoxicity Effects of Eryngium thyrsoideum Extracts on BC and Normal Cell Lines
4.2.1. The Results of Cytotoxicity on MCF-7 Cells
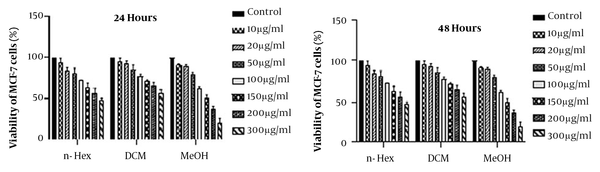
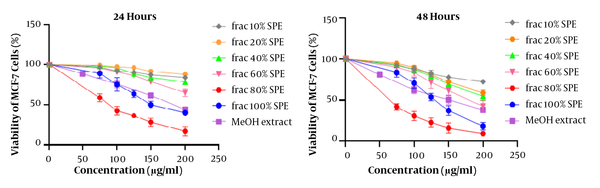
MTT assays are used to determine cytotoxicity and IC50. Figure 1 shows the data analysis of treated cells with n-Hex, MeOH, and DCM plant extracts at 10, 20, 50, 100, 150, 200, and 300 μg/mL concentrations and compared to the group in 24 and 48 hours. According to the findings, MeOH extract had a significant cytotoxicity on MCF-7 cancer cells (P < 0.001). Therefore, the cytotoxicity of MeOH extract’s fractions was investigated by MTT assay (Figure 2). To obtain IC50 values, the numbers obtained from the device adsorption in the MTT test were normalized to the DMSO control adsorption, and after transforming the concentrations into logarithmic values, nonlinear regression analysis was used. The IC50 values are indicated in Table 2. The results of different fractions of MeOH extract showed that only 80% and 100% SPE fractions had a remarkable cytotoxicity compared to the control.
| Sample | IC50 (μg/mL) | |
|---|---|---|
| 24 Hours of Treatment | 48 Hours of Treatment | |
| n-Hex extract | > 300 | > 300 |
| DCM extract | > 300 | > 300 |
| MeOH extract | 5.60± 184.00 | 5.39 ± 146.10 |
| MeOH extract fractions (%) | ||
| 10 | > 200 | > 200 |
| 20 | > 200 | > 200 |
| 40 | > 200 | > 200 |
| 60 | > 200 | 179.20 ± 7.34 |
| 80 | 89.12 ± 1.75 | 64.27 ± 2.66 |
| 100 | 159.30 ± 4.64 | 129.00 ± 5.03 |
IC50 Values of Extracts and MeOH Extract’s Fractions of Eryngium thyrsoideum on MCF-7 Cells
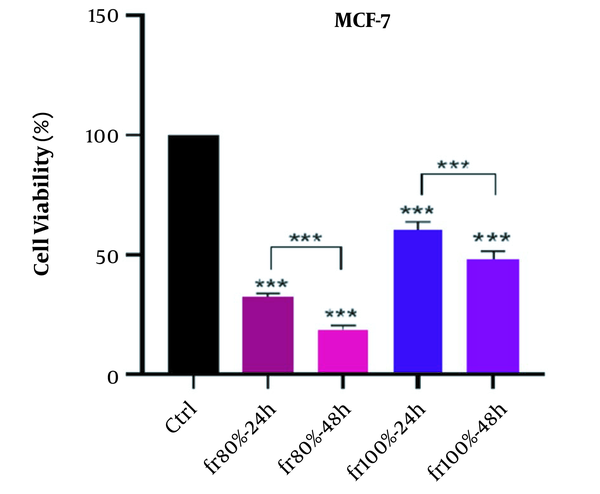
The time-dependent effects of MeOH extract and related fractions are presented in Figure 3. Our results showed that all plant samples had a time-dependent effect on the MCF-7 cell line and the effect of cytotoxicity increased significantly over time. In statistical evaluation, the significance level was determined as (P > 0.05), * (P < 0.05), ** (P < 0.01), *** (P < 0.001).
4.2.2. The Results of Cytotoxicity on MDA-MB-231 Cells
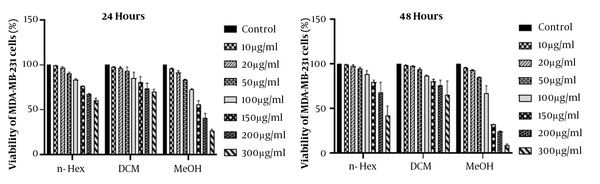
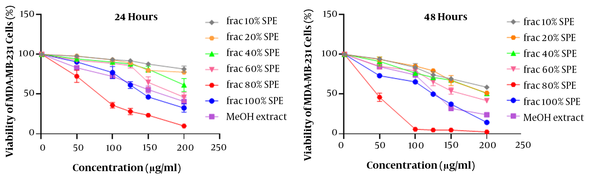
The results of MTT assay analysis are illustrated in Figure 4. Due to the significant cytotoxicity (P < 0.001) of MeOH extract on MDA-MB-231 cells compared to the DMSO control group, MeOH extract’s fractions were evaluated using MTT assay. The results of 24 and 48 hours are shown in Figure 5. The numbers obtained from the device adsorption in the MTT test were normalized to the DMSO control adsorption, and after transforming the concentrations into logarithmic values, nonlinear regression analysis was used to obtain IC50 values (Table 3). The results showed that MeOH extract and 80% and 100% SPE fractions had a significant cytotoxicity compared to the control. The effect of cytotoxicity on MDA-MB-231 cell line increased significantly with increasing treatment time (Figure 6).
| Sample | IC50 (μg/mL) | |
|---|---|---|
| 24 Hours of Treatment | 48 Hours of Treatment | |
| n-Hex extract | > 300 | 266.00±23.1 |
| DCM extract | > 300 | >300 |
| MeOH extract | 167.70 ± 9.39 | 127.30 ± 7.72 |
| MeOH extract fractions (%) | ||
| 10 | > 200 | > 200 |
| 20 | > 200 | > 200 |
| 40 | > 200 | > 200 |
| 60 | 189.60 ± 10.70 | 171.82 ± 11.95 |
| 80 | 78.46 ± 5.67 | 47.51 ± 3.72 |
| 100 | 148.70 ± 6.25 | 121.00 ± 3.02 |
IC50 Values of Extracts and MeOH Extract’s Fractions of Eryngium thyrsoideum on MDA-MB-231 Cells
4.2.3. The Results of Cytotoxicity on HFF-2 Cells
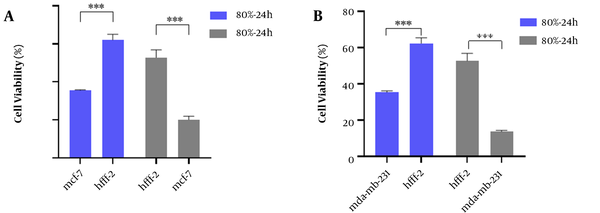
To evaluate the cytotoxicity effect of the strongest fraction (80% fraction compared to other fractions and control) of E. thyrsoideum on normal cell line (HFF-2), MTT test was performed. According to Figure 7, the viability of cancerous cell lines in 80% fraction was significantly reduced compared to normal HFF-2 cells. Therefore, this fraction had a selective cytotoxicity on cancer cell lines. IC50 values of 80% fraction on HFF-2 cells were 138.70 ± 4.22 and 126.10 ± 6.31 at 24 and 48 hours, respectively (Table 4).
| Sample | IC50 (μg/mL) | |
|---|---|---|
| 24 Hours of Treatment | 48 Hours of Treatment | |
| n-Hex extract | > 300 | > 300 |
| DCM extract | > 300 | > 300 |
| MeOH extract | 289.60 ± 10.72 | 271.82 ± 11.85 |
| MeOH extract fractions (%) | ||
| 10 | > 200 | > 200 |
| 20 | > 200 | > 200 |
| 40 | > 200 | > 200 |
| 60 | > 200 | > 200 |
| 80 | 126.10 ± 6.31 | 138.70 ± 4.22 |
| 100 | > 200 | > 200 |
IC50 Values of Extracts and MeOH Extract’s Fractions of Eryngium thyrsoideum on HFF-2 Cells
4.3. Evaluation of Apoptosis Rate in Treated Cancerous and Normal Cells
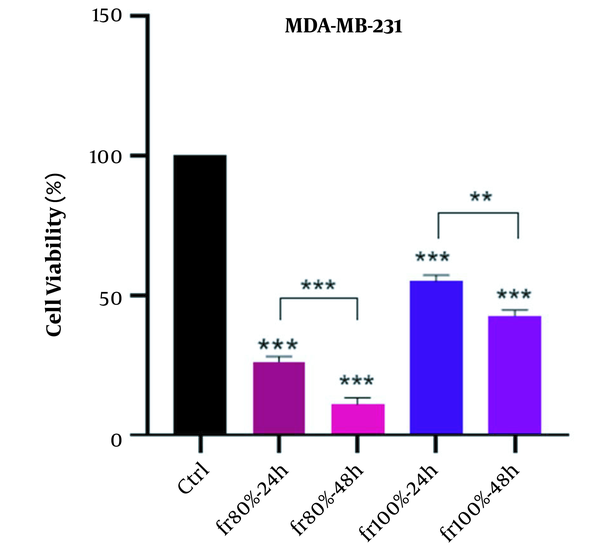
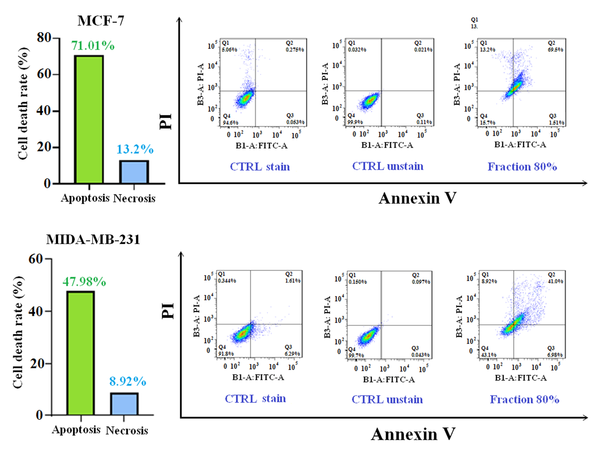
In this test, the strongest fraction of MeOH extract at IC50 concentration was incubated with cancer cell lines for 24 hours. Flow cytometry was carried out to detect the extent of apoptosis. The histogram view of the test by 80% fraction in both cancerous cell lines is shown in Figure 8. The results demonstrated that 80% fraction of MeOH extract can induce 71.1% apoptosis in MCF-7 cells and 47.98% in MDA-MB-231 cells. The number of apoptotic and necrotic cells is shown in Figure 8.
5. Discussion
Today, non-communicable diseases, like cancer, are the leading reason of death in the world. Cancers are one of the most common and spreading diseases with heavy economic costs for the international community. BC is still a common problem among women, despite many advances in distinction and treatment. Consequently, out of every four cases of cancer among women, one case is related to BC (33).
Due to the lack of optimal response, research on the production of drugs with greater efficiency and less toxicity is essential. Many chemotherapeutic and radiation therapies lack sufficient specificity on cancer cells and have side effects on other cells in the body. Hence, extensive studies are conducted around the world to achieve more effective and alternative therapies with fewer side effects. A significant part of these studies has targeted natural resources, including plants (34). There are many herbal compounds with a variety of biological effects that are part of the anti-cancer drugs in modern pharmaceutical science. Today, the number of plants recognized as potential anti-cancer agents is increasing day by day (35). Apoptosis plays a role as an essential cellular activity to maintain the body's physiological balance. Therefore, one of the most interesting strategies in chemotherapy is drug interventions that can mediate the death of pre-neoplastic or malignant cells by inducing apoptosis. In cellular studies, apoptosis is an important marker of the cytotoxic effects of anti-cancer compounds. There is ample evidence showing that there are many compounds in plants which induce their antitumor activity through apoptosis (36).
In this research, the cytotoxicity values of n-Hex, MeOH, and DCM extracts of E. thyrsoideum were evaluated on two BC cell lines (MCF-7 and MDA-MB-231) and HFF-2 cells as control cell line. For this aim, at the beginning of the study, cytotoxic effects and IC50 values were evaluated from the treated cell lines with various extracts. The results of cytotoxicity analysis showed that MeOH extract was significantly (P < 0.001) more toxic to both cancerous cell lines in 24- and 48- hour treatment than DMSO control. However, DCM and n-Hex extracts did not show a remarkable cytotoxic effect on these cells. Furthermore, based on our findings, the cytotoxicity of MeOH extract on both cancer cell lines was time-dependent (P < 0.01). The results of statistical analysis showed the notable decrease in cancerous viability by increasing the samples concentration.
In the continuation of the study, MeOH extract was chosen as the strongest extract, and its fractionation was done by SPE method. Statistical calculations showed that the 80% fraction had the greatest cytotoxicity compared to other fractions on each MCF-7 and MDA-MB-231 cell cycle. This fraction with P-value < 0.001 in both incubation times (24 and 48 hours) showed more cytotoxicity on cancer cell line than DMSO control. The results also indicated that the cytotoxic effects of 80% fraction against cancer cells are dose-dependent, and with increasing dose, the mortality rate of cancer cells increases. Furthermore, the effects of cytotoxicity of the mentioned fraction with P-value < 0.001 on the cancer cell lines are time-dependent. The results of statistical analysis demonstrated that the cytotoxicity effects depend on the concentration of MeOH extract and related fractions, and the cell viability was decreased by increasing the concentration of plant samples.
Dirani et al. indicated that E. creticum contains alkaloids, tannins, coumarins, saponins, flavonoids, and polyphenols that play a key role in cytotoxicity and apoptosis. In addition, ethanolic and aqueous extracts of the leaves of this plant have the greatest effect on cytotoxicity in HeLa cell line for 48 hours (37). Esmaeilli et al. evaluated the effects of cytotoxicity of 26 species grown in southwestern Iran on cancer cell line (A-549, HT-29, Hepa-2, and MCF-7). Four out of 26 species showed cytotoxic effects. The two species E. billardieri and Nerium indicum had the most cytotoxic effects (38). E. billardieri n-Hex and DCM extracts exerted their anti-proliferative effects on pancreatic cancer cell line (PANC-1) by inducing apoptosis via Caspases-3 and increasing Bax protein expression (13). Another study of total methanolic extracts of flowers, leaves, and stems of E. serbicum showed the effects of selective cytotoxicity on colorectal cancer cell lines HCT-116 and SW-480 and breast (MDA-MB-231 without affecting normal cells (MRC5)) (14). The results of cytotoxicity of E. campestre and E. amethystinum volatile oils on cancer cell line A375 (human malignant melanoma), MDA-MB-231, and HCT-116 cell line by MTT method showed high cytotoxicity (39).
In general, compounds in the genus Eryngium, such as essential oils, sterols, and saponins, might be responsible for anti-cell growth activity. Triterpene saponins responsible for the cytotoxicity of E. yuccifolium include Eryngiosides A-C, E, F, H-J, L, and Saniculasaponin III. Three eryngiosides derived from the genus Eryngium (eryngioside J, eryngioside L, and saniculasapoinin III) showed moderate cytotoxic properties against A-549, PC-3, HL-60, and MRC-5; they also significantly inhibited the growth of PANC cells. These compounds had non-specific action and cytotoxic effects on normal cell line (MRC-5). However, the two eryngiosides (H and I) showed more potent cytotoxicity effects on all cancer cell lines, without adverse effects on normal cell lines (40).
In this study, due to lack of access to normal human chemo-resistant BC cells (MCF-10A), cytotoxicity of plant samples was performed on normal human fibroblast cell line (HFF-2). The results of cytotoxicity on normal cells showed that the potent fraction of 80% in both incubation times (24 and 48 hours) between normal cell lines and two cancer cell lines acted selectively and had less adverse effects in normal cell line. According to the interpretation of flow cytometric data, the 80% fraction inhibits cancer cell growth by inducing apoptosis. The incidence of apoptosis with the mentioned fraction MCF-7 and MDA-MB-231 cell lines was estimated to be 71.1% and 47.98%, respectively. In addition, the 80% fraction with cytotoxicity, depending on concentration, time, and percentage of induction of apoptosis against both BC cell lines, created high hopes for the separation of effective compounds and clinical work in the treatment of BC in future studies.
5.1. Conclusions
According to our results, it can be concluded that the cytotoxic effects of E. thyrsoideum are related to MeOH extract. The 80% fraction had the highest cytotoxicity by the mechanism of apoptosis in cancerous cells, including MCF-7 and MDA-MB-231. On the other hand, this fraction had a selective cytotoxicity on cancer cells, and inhibition of cell growth increased with the incubation time (48 hours). Accordingly, it can be argued that cell growth inhibitory compounds derived from MeOH extract are concentrated in the 80% fraction. Therefore, 80% fraction of MeOH extract is the best candidate for future studies as a natural anti-cancer agent in BC therapy.