1. Background
The genus Scutellaria L., consisting of about 360 species around the world, is one of the largest genera classified in the Lamiaceae family (1). Some Scutellaria species are traditionally used as medicinal plants in different countries, including the root of Scutellaria baicalensis (Chinese skullcap) and the aerial part of Scutellaria barbata (barbed skullcap), which are well-known for their widespread therapeutic use in Chinese, Korean, and Japanese traditional medicines (2, 3).
A review of the literature suggests the pharmacological activities of some Scutellaria species, such as antioxidant, antibacterial, antiviral, hepatoprotective, neuroprotective, anticonvulsant, antitumor, and anti-angiogenesis effects (4, 5). Phytochemical studies have reported flavonoids, phenylethanoid glycosides, iridoids, and diterpenes as the main constituents of natural compounds found in Scutellaria species (4, 5). Moreover, the essential oils of most Scutellaria species have been reported to contain sesquiterpene hydrocarbons as their main constituents (6).
Iran, with 42 taxa, is one of the most important and diverse regions for the genus Scutellaria L. (7). Some Scutellaria taxa used in Iranian traditional medicine include Scutellaria lindbergii, Scutellaria litwinowii, Scutellaria luteo-caerulea, Scutellaria platystegia, and Scutellaria pinnatifida ssp. alpina (8). Scutellaria platystegia Juz. (Karabaghian skullcap) is an herbaceous perennial (up to 30 cm tall), which can be found as an Irano-Turanian species in northwestern Iran and the Transcaucasia region (7, 9). This species is differentiated from Scutellaria virens by the cordate base of its cauline leaves, as well as the long petiole (up to 20 mm) of its basal leaves (7).
2. Objectives
Regarding the pharmacological activities and bioactive compounds of Scutellaria species, the present study aimed to assess the antioxidant and antibacterial effects of the aerial part of S. platystegia and to identify the secondary metabolites present in its extract and essential oil.
3. Methods
3.1. Chemicals
Several chemicals were used in this study. Solvents, including n-hexane, chloroform, ethyl acetate, methanol, and acetone, were purchased from Samchun Chemical Co. (South Korea). Dimethyl sulfoxide, anhydrous sodium sulfate, silica gel 60 (mesh 230 - 400), and aluminum sheets, precoated with silica gel 60 F254, were purchased from Merck Co. (Germany). Moreover, a Sephadex LH-20 column was provided by GE Healthcare Co. (USA), while RP18 silica gel 60 (230 - 400 mesh), butylated hydroxytoluene, and 2,2-diphenyl-1-picrylhydrazyl were purchased from Sigma Aldrich Co. (USA).
The antimicrobial activity assays were conducted on the following bacterial strains, provided by the Iranian Research Organization for Science and Technology (IROST): Staphylococcus epidermidis (ATCC 12228), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 29737), Shigella dysenteriae (PTCC 1188), Klebsiella pneumonia (ATCC 10031), and Salmonella paratyphi serotype A (ATCC 5702). Additionally, antibiotics used in this study, including gentamicin, tetracycline, and rifampin, were purchased from Padtan Teb Co. (Iran). The microbial media, brain-heart infusion (BHI) broth, nutrient ager (NA), tryptic soy broth (TBS), Sabouraud dextrose agar (SDA), and Mueller-Hinton agar (MHA) were obtained from Merck Co. (Germany).
3.2. Plant Material
The aerial part of S. platystegia Juz. was collected at its flowering stage from Jolfa (East Azerbaijan, Iran) in June 2017. Authentication of plant samples was performed by a taxonomist (Dr. Yousef Ajani). They were assigned a PMP-378 code at the herbarium of Tehran University of Medical Sciences, Tehran, Iran.
3.3. Extraction and Fractionation
For this purpose, 800 g of air-dried and comminuted aerial part of S. platystegia was macerated four times with 70% methanol in water (5 L of each) within 72-hour intervals at room temperature. The extract was then concentrated using a rotary evaporator at 40°C and then dried in a vacuum oven. The resultant hydroalcoholic extract was suspended in water (1 L) and fractionated using n-hexane, chloroform, and n-butanol (4 × 1 L each) successively. The obtained fractions were concentrated using a rotary evaporator (40°C), and the solvents were completely removed in a vacuum oven (40°C).
3.4. Antioxidant Activity
The antioxidant effects of n-hexane, chloroform, and n-butanol fractions were investigated using the ferric reducing ability of plasma (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assays.
3.4.1. DPPH Free Radical Scavenging Assay
For this purpose, 1 mL of DPPH solution (80 mg/mL in methanol) was mixed in 1 mL of sample solutions, prepared at concentrations ranging from 1.0 to 3.9 × 10-3 mg/mL in methanol. The samples were kept in the dark at 25°C for 30 minutes, and absorption was measured at 517 nm using an Optizen 2120UV PLUS spectrophotometer (Mecasys Co., Korea) (10). Butylated hydroxytoluene (BHT) was used as the positive control. The tests were repeated three times. The half maximal inhibitory concentration (IC50; μg/mL) is expressed as mean ± SD.
3.4.2. FRAP Assay
The FRAP method was used to evaluate the reducing power of the main fractions (11). Briefly, 5 mL of TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mM in 40 mM HCl), 50 mL of acetate buffer (0.3 M, pH = 3.6), and 5 mL of a freshly prepared FeCl3 solution (20 mM) were mixed to prepare the FRAP reagent. To measure the reducing power, 100 μL of the sample solution (500 μg/mL in DMSO) was moved to a test tube, containing 3 mL of FRAP reagent; the mixture was then incubated at 37°C for 10 minutes. The UV absorption was then recorded at 593 nm. Ferrous sulfate heptahydrate (FeSO4.7H2O) solutions (125 - 1,000 μM) were used to draw a calibration curve; vitamin E, as a natural antioxidant, was also used as the positive control. All the tests were performed in triplicate; the results are expressed as mmol FeSO4.7H2O equivalent per gram of tested sample (mmol FSE/g).
3.5. Antibacterial Activity
The following methods were applied to evaluate the antibacterial activities of n-hexane, chloroform, and n-butanol fractions from the total hydroalcoholic extract of S. platystegia.
3.5.1. Disk Diffusion Assay
The solutions were filtered through 0.45-μm Millipore filters. The solution of each fraction (10 μL) was placed on discs (6 mm in diameter), containing MHA plates, inoculated with the bacterial suspension. The inhibition zones (IZ, mm) were measured in diameter following incubation at 37°C. Rifampin (5 μg/disc) and gentamicin (10 μg/disc) were the positive controls used in this study (12).
3.5.2. Microdilution Assay
The minimum inhibitory concentrations (MICs) of the fractions were determined by the microdilution assay. For this purpose, 95 μL of the culture medium, 5 μL of the bacterial inoculum, and 100 μL of the sample solution were added to the wells of 96-well plates. The prepared mixture, without the sample solution, was considered as the negative control. Gentamicin and rifampin were also used as the positive controls. The wells were shaken using a plate shaker at 300 rpm for 30 seconds and then incubated at their optimal growth temperature for 24 hours (13).
3.6. Isolation and Purification of Compounds
The chloroform fraction (6.0 g) was subjected to normal phase silica gel column chromatography (4 × 70 cm) and eluted with a gradient solvent system of CHCl3-EtOAc (90:10% to 60:40%) to obtain 16 fractions (C1-C16). Compounds 1 and 2 (5.5 mg) at a ratio of 5.5:4.5, as well as compound 3 (8.2 mg), were extracted from fraction C3 (145 mg) through silica gel column chromatography (2 × 70 cm) with n-hexane-EtOAc (80:20% to 50:50%). The chromatography of C2 (145 mg) and C11 (145 mg) fractions on a Sephadex LH-20 column (1.5 × 70 cm), with MeOH-EtOAc (80:20) as the mobile phase, resulted in the isolation of compound 4 (6.4 mg) and compound 5 (5.5 mg), respectively. A portion of n-butanol fraction (10 g) was also subjected to Sephadex LH-20 column chromatography (5 × 25 cm) with MeOH-H2O (80:20) and divided into eight subfractions (B1-B8).
The re-chromatography of fraction B3 (463 mg) on the Sephadex LH-20 column (2 × 70 cm) with methanol led to the isolation of compounds 6 (12.7 mg), 7 (24.3 mg), 8 (11.8 mg), and 9 (15.1 mg). Fraction B4 (279 mg) was added to the Sephadex LH-20 column (2 × 70 cm) and eluted with methanol to obtain compounds 10 (7.6 mg), 11 (13.2 mg), 12 (16.8 mg), and 13 (10.5 mg). These compounds were individually purified on a reverse phase (C18) column (1 × 20 cm), using ACN-H2O (50:50% to 80:20%). Compound 14 (14.8 mg) was also isolated from fraction B8 (51 mg) via Sephadex LH-20 column chromatography (5 × 25 cm), with MeOH-H2O (80:20) as the solvent system. Thin layer chromatography (TLC) was used for monitoring column chromatography; fractions with similar spots under UV light (254 and 366 nm) were combined. The chemical structures of the isolated compounds were characterized by nuclear magnetic resonance (NMR) imaging (1H NMR and 13C NMR, Bruker Avance 300 DRX) and UV-Vis spectral analysis (using an Optizen 2120UV PLUS spectrophotometer), in addition to comparison with the published data.
3.7. Essential Oil Extraction
The hydrodistillation method was performed using a Clevenger-type apparatus to extract essential oil from the powdered plant material (100 g). After three hours, the produced oil was isolated and dried with anhydrous sodium sulfate and stored at -20°C until further gas chromatography–mass spectrometry (GC-MS) analysis.
3.8. GC-MS Analysis
A GC-MS analysis of the plant essential oil was carried out on an HP-6890 gas chromatograph, equipped with an HP-5973 mass detector via electron impact ionization (70 eV). An HP-5MS column (30 m × 0.25 mm; film thickness, 0.25 μm) was used for this purpose. The flow of helium as the carrier gas was 1 mL/min. Analysis was carried out in an isotherm oven at 60°C for three minutes, increasing from 60°C to 250°C at a rate of 3°C/min for 65 minutes. The temperature of the injector and detector was held constant at 220°C and 290°C, respectively. The injection volume was 1.0 μL, and the split ratio was 1:90. The Kovats retention index (KI) was calculated using a homologous series of n-alkanes (C8-C24). Besides, the essential oil constituents were identified based on the mass spectrum matching of the constituents with those recorded for standard compounds, as well as comparison of KI indices.
4. Results
The results of DPPH and FRAP assays for evaluating the antioxidant activity of the main fractions of the hydroalcoholic extract of S. platystegia are summarized in Table 1. In both tests, n-butanol fraction was the most potent fraction, with an IC50 of 16.14 ± 0.8 µg/mL on the DPPH test and 736.4 ± 4.6 mmol FeSO4.7H2O equivalent/g of sample on the FRAP assay; the results were comparable to the results obtained for vitamin E as the positive control (IC50 = 14.8 ± 3.3 µg/mL on the DPPH test and 127.8 ± 4.1 mmol FeSO4.7H2O equivalent/g on the FRAP assay).
| Samples | DPPH Assay (IC50, µg/mL) | FRAP Assay (mmol FeSO4.7H2O Equivalent/g) |
|---|---|---|
| n-Hexane fraction | 22.01 ± 1.2 | 330.4 ± 2.0 |
| Chloroform fraction | 75.70 ± 3.3 | 358.4 ± 3.5 |
| n-Butanol fraction | 16.14 ± 0.8 | 736.4 ± 4.6 |
| Vitamin E | 14.8 ± 3.3 | 127.8 ± 4.1 |
Antioxidant Activity of Major Fractions Obtained from the Hydroalcoholic Extract of the Aerial Part of Scutellaria platystegiaa
The growth inhibitory effects of n-hexane, chloroform, and n-butanol fractions against the selected bacterial strains are presented in Table 2. As shown in Table 2, although the tested samples were not active against B. subtilis and K. pneumoniae, they demonstrated moderate (IZ, 10 - 15 mm) to very strong (IZ > 20 mm) growth inhibitory activities against four other bacterial strains (Table 2). Among the tested fractions, n-butanol showed very strong antibacterial activities against S. aureus (IZ: 25 mm, MIC: 125 µg/mL), S. epidermidis (IZ: 21 mm, MIC: 125 µg/mL), and S. dysenteriae (IZ: 20 mm, MIC: 250 µg/mL). The zone of inhibition was the largest for the n-butanol, chloroform, and n-hexane fractions, respectively.
| Microorganisms | n-Hexane Fraction | Chloroform Fraction | n-Butanol Fraction | Rifampin (5 μg/disc) | Gentamicin (10 μg/disc) |
|---|---|---|---|---|---|
| Salmonella paratyphi A | 10 b (500) c | 16 (500) | 17 (500) | - | 21 (500) |
| Klebsiella pneumoniae | - | - | - | 7 (250) | 22 (250) |
| Staphylococcus aureus | 11 (500) | 18 (250) | 25 (125) | 10 (250) | 21 (500) |
| Staphylococcus epidermidis | 10 (500) | 15 (250) | 21 (125) | 8 (250) | 18 (500) |
| Bacillus subtilis | - | - | - | 13 (15) | 21 (500) |
| Shigella dysenteriae | 12 (250) | 17 (250) | 20 (250) | 40 (250) | 35 (500) |
Antibacterial Activities of the Main Fractions Obtained from the Hydroalcoholic Extract of the Aerial Part of Scutellaria platystegiaa
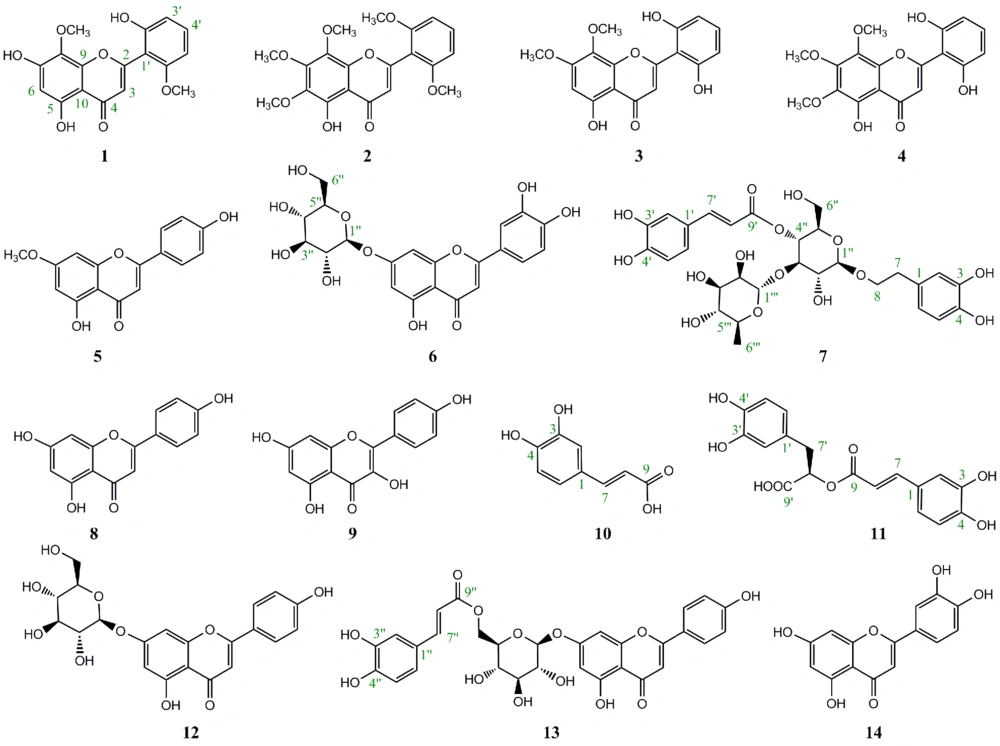
The phytochemical analysis of the aerial part of S. platystegia via normal and reversed phase column chromatography on silica gel and Sephadex LH-20 column led to the isolation of five compounds (compounds 1 - 5) from the chloroform fraction and nine compounds (compounds 6 - 14) from the n-butanol fraction. The chemical structures of the isolated compounds were identified: 5,7,2’-trihydroxy-8,6’-dimethoxyflavone (14), 5-hydroxy-6,7,8,2’,6’-pentamethoxyflavone (15), 5,2’,6’-trihydroxy-7,8-dimethoxyflavone (16, 17), 5,2’,6’-trihydroxy-6,7,8-trimethoxyflavone (18), 5,4’-dihydroxy-7-methoxyflavone (19, 20), luteolin 7-O-β-D-glucopyranoside (21), verbascoside (21), apigenin (22), kaempferol (23, 24), caffeic acid (25, 26), rosmarinic acid (27, 28), apigenin-7-O-β-D-glucopyranoside (21, 22), apigenin-7-O-β-D-(-6’’-(E)-caffeoyl)-glucopyranoside (29), and luteolin (28, 30), using spectroscopic methods, including NMR (1H-NMR and 13C-NMR) and UV-Vis analysis, as well as comparison with previously published data (Figure 1).
4.1. NMR Data of the Isolated Compounds
Compound 1: 5,7,2’-trihydroxy-8,6’-dimethoxyflavone (C17H14O7); 1H-NMR (CDCl3, 300 MHz): δ 12.41 (1H, s, OH-5), 7.32 (1H, t,J=8.2 Hz, H-4’), 6.70 (1H, s, H-3), 6.66 (1H, d,J=8.2 Hz, H-5’), 6.56 (1H, d,J=8.2 Hz, H-3’), 6.42 (1H, s, H-6), 3.94 (3H, s, OCH3), and 3.84 (3H, s, OCH3), respectively; 13C-NMR (CDCl3, 75 MHz): δ 182.84 (C-4), 164.50 (C-2), 161.30 (C-2’), 160.51 (C-6’), 154.93 (C-7), 154.85 (C-5), 153.31 (C-9), 131.77 (C-4’), 128.79 (C-8), 109.57 (C-5’), 106.93 (C-3), 105.93 (C-1’), 103.87 (C-3’), 102.94 (C-10), 98.82 (C-6), 60.64 (OCH3), and 56.77 (OCH3), respectively.
Compound 2: 5-hydroxy-6, 7,8,2’,6’-pentamethoxyflavone (C20H20O8); 1H-NMR (CDCl3, 300 MHz): δ 12.35 (1H, s, OH-5), 7.32 (1H, t,J= 8.2 Hz, H-4’), 6.71 (1H, s, H-3), 6.66 (1H, d,J= 8.2 Hz, H-5’), 6.56 (1H, d,J= 8.2 Hz, H-3’), 4.10 (3H, s, OCH3), 3.94 (3H, s, OCH3), 3.91 (3H, s, OCH3), 3.87 (3H, s, OCH3), and 3.84 (3H, s, OCH3), respectively; 13C-NMR (CDCl3, 75 MHz): δ 182.27 (C-4), 163.88 (C-2), 160.64 (C-2’, C-6’), 150.45 (C-9), 150.15 (C-5), 149.80 (C-7), 132.54 (C-6), 131.16 (C-8), 129.64 (C-4’), 111.40 (C-1’), 107.31 (C-3), 105.24 (C-3’), 105.24 (C-5’), 104.50 (C-10), 60.64 (OCH3), 60.64 (OCH3), 60.64 (OCH3), 56.78 (OCH3), and 56.78 (OCH3), respectively.
Compound 3: 5,2’,6’-trihydroxy-7,8-dimethoxyflavone (viscidulin II) (C17H14O7); 1H-NMR (CDCl3, 300 MHz): δ 12.42 (1H, s, OH-5), 7.33 (1H, t,J=8.3 Hz, H-4’), 6.65 (1H, d,J=8.3 Hz, H-5’), 6.58 (1H, d,J=8.3 Hz, H-3’), 6.55 (1H, s, H-3), 6.43 (1H, s, H-6), 3.94 (3H, s, OCH3), and 3.82 (3H, s, OCH3), respectively; 13C-NMR (CDCl3, 75 MHz): δ 182.85 (C-4), 164.61 (C-2), 160.15 (C-2’), 160.15 (C-6’), 159.10 (C-7), 156.91 (C-5), 151.37 (C-9), 132.22 (C-4’), 130.53 (C-9), 108.33 (C-3’), 108.33 (C-5’), 108.16 (C-3), 104.80 (C-10), 104.68 (C-1’), 97.45 (C-6), 60.64 (OCH3), and 56.77 (OCH3), respectively.
Compound 4: 5,2’,6’-trihydroxy-6,7,8-trimethoxyflavone (C18H16O8); 1H-NMR (CDCl3, 300 MHz): δ 12.57 (1H, s, OH-5), 7.32 (1H, t,J=8.3 Hz, H-4’), 6.66 (1H, s, H-3), 6.65 (1H, d,J=8.3 Hz, H-5’), 6.57 (1H, d,J=8.2 Hz, H-3’), 4.03 (3H, s, OCH3), 3.95 (3H, s, OCH3), and 3.83 (3H, s, OCH3), respectively; 13C-NMR (CDCl3, 75 MHz): δ 182.29 (C-4), 164.63 (C-2), 160.17 (C-2’), 160.17 (C-6’), 150.46 (C-9), 150.17 (C-5), 149.82 (C-7), 132.55 (C-6), 132.24 (C-4’), 131.17 (C-8), 108.35 (C-3’), 108.35 (C-5’), 108.17 (C-3), 104.69 (C-1’), 104.52 (C-10), 60.65 (OCH3), 60.65 (OCH3), and 60.65 (OCH3), respectively.
Compound 5: 5,4’-dihydroxy-7-methoxyflavone (genkwanin) (C16H12O5); 1H-NMR (CDCl3, 300 MHz): δ 12.51 (1H, s, OH-5), 7.92 (2H, d,J=7.4 Hz, H-2’,6’), 7.57 (1H, s, H-3), 7.56 (2H, d,J=7.4 Hz, H-3’,5’), 6.69 (1H, br s, H-8), 6.45 (1H, br s, H-6), and 4.05 (3H, s, OCH3), respectively; 13C-NMR (CDCl3, 75 MHz): δ 182.25 (C-4), 166.41 (C-2), 166.04 (C-7), 161.67 (C-4’), 161.67 (C-9), 158.45 (C-5), 129.01 (C-2’), 129.01 (C-6’), 122.32 (C-1’), 116.08 (C-3’), 116.08 (C-5’), 105.69 (C-10), 104.77 (C-3), 99.01 (C-6), 93.62 (C-8), and 55.09 (OCH3), respectively.
Compound 6: Luteolin-7-O-β-D-glucopyranoside (C21H20O11); 1H-NMR (DMSO-d6, 300 MHz): δ 7.42 (1H, br d,J=8.6, H-5’), 7.41 (1H, br s, H-2’), 6.88 (1H, d,J=8.6 Hz, H-6’), 6.76 (1H, d,J=1.6, H-8), 6.73 (1H, s, H-3), 6.40 (1H, d,J=1.6, H-6), 6.53 (1H, s, H-3), 5.07 (1H, d,J=7.1 Hz, H-1”), and 3.1 - 4.0 (3H, overlapped signals, H-2” to H-6”), respectively. 13C-NMR (DMSO-d6, 75 MHz): δ 182.1 (C-4), 163.8 (C-2), 162.8 (C-7), 161.2 (C-5), 157.0 (C-9), 149.8 (C4’), 145.8 (C-3’), 121.4 (C-1’), 120.2 (C-6’), 116.0 (C-5’), 113.5 (C-2’), 105.1 (C-10), 103.2 (C-3), 99.6 (C-6), 95.0 (C-8), 98.0 (C1”), 78.4 (C-2”), 77.1 (C-5”), 75.8 (C-3”), 69.6 (C-4”), and 60.6 (C-6”), respectively.
Compound 7: Verbascoside (C29H36O15); 1H-NMR (DMSO-d6, 300 MHz): δ 7.44 (1H, d,J=15.8 Hz, H-7’), 7.01 (1H, d,J=1.6 Hz, H-2’), 6.96 (1H, dd,J=8.3, 1.6 Hz, H-6’), 6.75 (1H, d,J=8.3 Hz, H-5’), 6.62 (1H, d,J=8.1 Hz, H-5), 6.62 (1H, d,J=1.7 Hz, H-2), 6.47 (1H, dd,J=8.1, 1.7 Hz, H-6), 6.20 (1H, d,J=15.8 Hz, H-8’), 5.01 (1H, br s, H-1’’’), 4.69 (1H, t,J=9.6 Hz, H-4’’), 4.34 (1H, d,J=7.7 Hz, H-1’’), 3.07-3.89 (10H, overlapped signals, H-2’’, H-3’’, H-5’’, H-6’’, H-2’’’ to H-5’’’ and H-8), 2.68 (2H, m, H-7), and 0.94 (3H, d,J=6.0 Hz, H-6’’’), respectively; 13C-NMR (DMSO-d6, 75 MHz): δ 166.24 (C-9’), 148.68 (C-4’), 146.05 (C-7’), 145.79 (C-3’), 145.18 (C-3), 143.73 (C-4), 129.69 (C-1), 125.98 (C-1’), 122.07 (C-6’), 120.10 (C-6), 116.67 (C-2), 116.19 (C-8’), 115.88 (C-5), 114.88 (C-2’), 114.05 (C-5’), 102.68 (C-1’’), 101.68 (C-1’’’), 79.66 (C-3’’), 74.85 (C-2’’), 74.85 (C-5’’), 71.96 (C-4’’’), 70.84 (C-8), 70.69 (C-2’’’), 70.65 (C-3’’’), 69.57 (C-2’’), 69.18 (C-5’’), 61.04 (C-6’’), 35.40 (C-7), and 18.56 (C-6’’’), respectively.
Compound 8: Apigenin (C15H10O5): 1H-NMR (DMSO-d6, 300 MHz): δ 12.60 (1H, s, OH-5), 8.17 (2H, d,J=8.1 Hz, H-2’,6’), 7.59 (1H, s, H-3), 7.58 (2H, d,J=8.1 Hz, H-3’,5’), 7.00 (1H, br s, H-8), and 6.30 (1H, br s, H-6), respectively; 13C-NMR (DMSO-d6, 75 MHz): 182.38 (C-4), 164.87 (C-2), 164.50 (C-7), 162.17 (C-4’), 161.85 (C-5), 158.02 (C-9), 129.23 (C-2’), 129.23 (C-6’), 121.26 (C-1’), 116.74 (C3’), 116.74 (C-5’), 104.31 (C-10), 103.47 (C-3), 99.58 (C-6), and 94.76 (C-8), respectively.
Compound 9: Kaempferol (C15H10O6); 1H-NMR (DMSO-d6, 300 MHz): δ 12.73 (1H, s, OH-5), 8.00 (2H, d,J=8.1 Hz, H-2’,6’), 6.91 (2H, d,J=8.1 Hz, H-3’,5’), 6.78 (1H, br s, H-8), 6.25 (1H, br s, H-6); 13C-NMR (DMSO-d6, 75 MHz): δ 177.79 (C-4), 165.68 (C-7), 162.60 (C-5), 160.72 (C-4’), 158.45 (C-9), 148.21 (C-2), 137.73 (C-3), 130.67 (C-2’), 130.67 (C-6’), 123.82 (C-1’), 116.46 (C-3’), 116.46 (C-5’), 104.73 (C-10), 99.41 (C-6), and 94.28 (C-8), respectively.
Compound 10: Caffeic acid (C9H8O4); 1H-NMR (DMSO-d6, 300 MHz): δ 7.33 (1H, d,J=15.7 Hz, H-7), 6.98 (1H, br s, H-2), 6.90 (1H, br d,J=8.1, H-6), 6.72 (1H, d,J=8.1 Hz, H-5), and 6.14 (1H, d,J=15.7 Hz, H-8), respectively; 13C-NMR (DMSO-d6, 75 MHz): δ 170.17 (C-9), 150.25 (C-4), 149.62 (C-3), 146.33 (C-7), 128.36 (C-1), 125.10 (C-6), 118.42 (C-8), 117.54 (C-5), and 115.13 (C-2), respectively.
Compound 11: Rosmarinic acid (C18H16O8); 1H-NMR (DMSO-d6, 300 MHz): δ 7.36 (1H, d,J=15.8 Hz, H-7), 7.01 (1H, br s, H-2), 6.91 (1H, br d,J=8.4, H-6), 6.72 (1H, d,J=8.4 Hz, H-5), 6.64 (1H, br s, H-2’), 6.58 (1H, d,J=8.2 Hz, H-5’), 6.47 (1H, dd,J=8.2, 1.4 Hz, H-6’), 6.15 (1H, d,J=15.8 Hz, H-8), 4.83 (1H, dd,J=9.7, 4.5, H-8’), 2.98 (1H, dd,J=14.7, 2.7 Hz, H-7’a), and 2.84 (1H, dd,J=14.3, 10.0 Hz, H-7’b), respectively; 13C-NMR (DMSO-d6, 75 MHz): δ 171.49 (C-9’), 166.45 (C-9), 149.04 (C-3), 146.19 (C-7), 146.04 (C-4), 145.36 (C-4’), 144.35 (C-3’), 128.06 (C-1’), 125.83 (C-1), 122.06 (C-6), 120.48 (C-5’), 117.10 (C-2’), 116.23 (C-5), 115.84 (C-6’), 115.25 (C-2), 113.89 (C-8), 72.93 (C-8’), and 36.63 (C-7’), respectively.
Compound 12; Apigenin-7-O-β-D-glucopyranoside (C21H20O10); 1H-NMR (DMSO-d6, 300 MHz): δ 7.95 (2H, d,J=8.5 Hz, H-2’,6’), 6.93 (2H, d,J=8.5 Hz, H-3’,5’), 6.80 (1H, d,J=2.1 Hz, H-8), 6.71 (1H, s, H-3), 6.61 (1H, d,J=2.1 Hz, H-6), 4.95 (1H, d,J=7.3 Hz, H-1”), 3.1-4.0 (3H, H-2”-6”); 13C-NMR (DMSO-d6, 75 MHz): δ 182.36 (C-4), 164.70 (C-2), 163.27 (C-7), 161.61 (C-5), 161.02 (C-4’), 157.38 (C-9), 129.05 (C-2’), 129.05 (C-6’), 121.42 (C-1’), 116.40 (C-3’), 116.40 (C-5’), 105.74 (C-10), 103.53 (C-3), 100.28 (C-1’’), 99.92 (C-6), 95.36 (C-8), 77.46 (C-3’’), 76.57 (C-5’’), 73.36 (C-2’’), 69.90 (C-4’’), and 60.94 (C-6’’), respectively.
Compound 13: Apigenin-7-O-β-D-(-6’’-(E)-caffeoyl)-glucopyranoside (C30H26O13); 1H-NMR (DMSO-d6, 300 MHz): δ 7.89 (2H, d,J= 8.7 Hz, H-2’ and H-6’), 7.45 (1H, br s, H-2’’’), 7.42 (1H, dd,J=8.3, 1.6 Hz, H-6’’’), 7.39 (1H, d,J=15.8, H-7’’’), 6.92 (2H, d,J=8.7 Hz, H-3’ and H-5’), 6.87 (1H, br s, H-8), 6.86 (1H, d,J=8.3 Hz, H-5’’’), 6.71 (1H, s, H-3), 6.61 (1H, br s, H-6), 6.20 (1H, d,J=15.8, H-8’’’), 5.08 (1H, d,J=7.3, H-1’’), 4.45 (1H, dd,J=11.1, 2.1 Hz, H-6’’a), 4.21 (1H, dd,J=11.2, 6.5 Hz, H-6’’b), 3.82 (1H, m, H-5’’), and 3.1 - 3.6 (3H, overlapped signals, H-2’’ to H-4’’), respectively; 13C-NMR (DMSO-d6, 75 MHz): δ 184.1 (C-4), 168.7 (C-9’’’), 166.6 (C-2), 163.6 (C-4’), 162.7 (C-5), 164.4 (C-7), 158.8 (C-9), 149.5 (C-4’’’), 147.1 (C-3’’’), 146.5 (C-8’’’), 129.3 (C-2’), 129.3 (C-6’), 126.7 (C-1’’’), 122.7 (C-6’’’), 122.1 (C-1’), 117.0 (C-3’), 117.0 (C-5’), 115.1 (C-2’’’), 116.5 (C-5’’’), 114.4 (C-7’’’), 106.5 (C-10), 103.6 (C-3), 101.1 (C-1’’), 101.0 (C-6), 95.4 (C-8), 77.6 (C-3’’), 75.4 (C-5’’), 74.1 (C-2’’), 71.8 (C-4’’), and 64.7 (C-6’’), respectively.
Compound 14: Luteolin (C15H10O6): 1H-NMR (DMSO-d6, 300 MHz): δ 12.97 (1H, s, OH-5), 7.40 (1H, br d,J=8.1 Hz, H-6’), 7.38 (2H, br s, H-2’), 6.91 (1H, d,J=8.1 Hz, H-5’), 6.42 (1H, br s, H-8), and 6.16 (1H, br s, H-6), respectively; 13C-NMR (DMSO-d6, 75 MHz): δ 181.9 (C-4), 164.3 (C-7), 164.0 (C-2), 161.5 (C-5), 157.3 (C-9), 149.8 (C-4’), 145.9 (C-3’), 121.7 (C-1’), 119.1 (C-6’), 116.3 (C-5’), 113.5 (C-2’), 103.7 (C-10), 102.8 (C-3), 98.5 (C-6), and 94.0 (C-8), respectively.
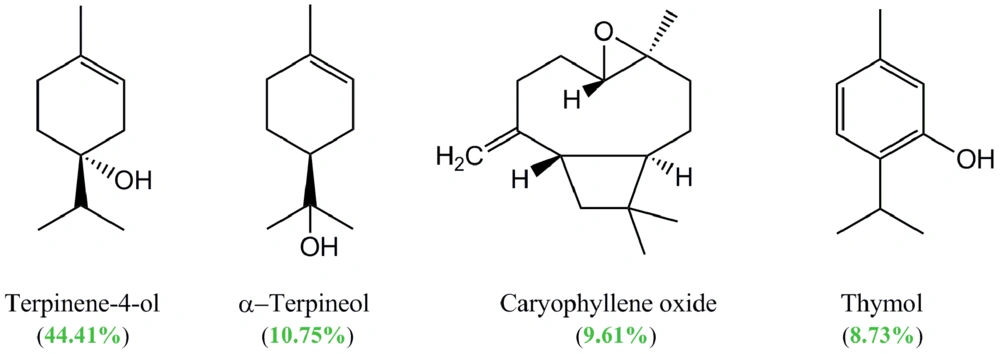
In this study, the essential oil composition of the aerial part of S. platystegia, extracted by the hydrodistillation method (yield: 0.1 % [v/w]), was also analyzed. The GC-MS analysis of the essential oil extracted from the plant aerial part resulted in the identification of 14 compounds, comprising 97.85% of the oil (Table 3). Among the identified compounds, terpinen-4-ol (44.41%), α-terpineol (10.75%), caryophyllene oxide (9.61%), and thymol (8.73%) were the main compounds (Figure 2). In the analyzed essential oil, oxygenated monoterpenes with a relative percentage of 74.28% comprised the main group of constituents. The GC chromatogram of S. platystegia essential oil can be found in Appendix 1 in the supplementary file.
| No. | Compounds | RT | Relative Area% | KI |
|---|---|---|---|---|
| 1 | α-Pinene | 8.81 | 2.76 | 939 |
| 2 | p-Cymene | 12.08 | 1.87 | 1032 |
| 3 | γ-Terpinene | 13.38 | 1.65 | 1062 |
| 4 | α-Terpinolene | 14.60 | 0.67 | 1083 |
| 5 | Linalool | 15.37 | 1.75 | 1085 |
| 6 | trans-2-Menthenol | 16.46 | 2.03 | 1146 |
| 7 | cis-Verbenol | 17.72 | 2.36 | 1160 |
| 8 | Terpinen-4-ol | 19.00 | 44.41 | 1177 |
| 9 | α-Terpineol | 19.85 | 10.75 | 1189 |
| 10 | Verbenone | 20.38 | 4.25 | 1207 |
| 11 | Thymol | 25.98 | 8.73 | 1290 |
| 12 | Trans-Caryophyllene | 29.14 | 4.75 | 1420 |
| 13 | Bicyclogermacrene | 32.67 | 2.26 | 1490 |
| 14 | Caryophyllene oxide | 37.76 | 9.61 | 1570 |
| Monoterpene hydrocarbons | 6.95 | |||
| Oxygenated monoterpenes | 74.28 | |||
| Sesquiterpene hydrocarbons | 7.01 | |||
| Oxygenated hydrocarbons | 9.61 | |||
| Total | 97.85 |
Chemical Composition of the Essential Oil of Scutellaria platystegiaa
5. Discussion
Today, the role of oxidative stress is well established in the pathogenesis of some diseases, such as diabetes, cancer, and inflammatory or neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases (31). Plants are sources of natural antioxidants, and their use as herbal supplements or herbal teas can be helpful to prevent oxidative stress-related diseases (32). In the present study, different fractions obtained from the hydroalcoholic extract of the aerial part of S. platystegia were assessed for their antioxidant effects. The n-butanol fraction was found to be the most potent fraction on DPPH (IC50 = 16.14 ± 0.8 µg/mL) and FRAP (736.4 ± 4.6 mmol FeSO4.7H2O equivalent/g) assays (Table 1).
In 2010, Senol et al. screened the antioxidant activity of 33 Scutellaria taxa from Turkey and found the methanol extract of S. orientalis L. subsp. pinnatifida J.R.Edm. to be the most potent sample on the DPPH (percentage of DPPH radical inhibition at 250 μg/mL: 87.62%) and FRAP (700 nm absorbance at 250 μg/mL = 0.313) assays (33). In another study, Salini et al. revealed that the chloroform extracts of Scutellaria colebrookiana and Scutellaria violacea had significant protective effects against 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative damage in human erythrocytes, with IC50 of 18.3 and 23.5 µg/mL, respectively (34). Recently, some mechanistic animal studies have demonstrated that baicalin, a major flavonoid glycoside found in S. baicalensis roots, can alleviate atherosclerosis (35), lipopolysaccharide-induced acute lung damage (36), deoxynivalenol-induced intestinal injury (37), and chronic mild stress-induced depression-like behaviors by relieving different oxidative stress and inflammatory responses (38).
In the present study, the n-butanol fraction of S. platystegia showed strong to very strong antibacterial activity against S. paratyphi A (IZ: 17 mm, MIC: 500 µg/mL), S. aureus (IZ: 25 mm, MIC: 125 µg/mL), S. epidermidis (IZ: 21 mm, MIC: 125 µg/mL), and S. dysenteriae (IZ: 20 mm, MIC: 250 µg/mL) (Table 1). Besides, the chloroform faction demonstrated strong inhibitory activity against the growth of the mentioned bacteria, with an inhibition zone diameter ranging from 15 mm to 18 mm (Table 1). Moreover, a review of previous studies indicated the antimicrobial activities of some Scutellaria species (38-41). In this regard, Fazly Bazzaz et al. (2011 and 2013) tested the antimicrobial potential of different extracts of S. lindbergii and S. litwinowii against E. coli, P. aeruginosa, S. aureus, B. cereus, and C. albicans and found the ethyl acetate and methanol extracts to be the most active extracts against S. aureus, with MIC of 6.25 mg/mL (39, 40).
Additionally, in a study on the antibacterial activity of 17 Scutellaria orientalis taxa growing in Turkey, Yilmaz et al. reported moderate to good antimicrobial activity (MIC: 62.5 - 250 μg/mL) for the methanol extracts of all tested taxa, including S. orientalis subsp. santolinoides, S. orientalis subsp. orientalis, and S. orientalis subsp. haussknechtii as the most effective ones (41). Also, Rajendran et al. investigated the antibacterial activity of some flavonoids isolated from the acetone extract of Scutellaria oblonga against 11 foodborne pathogens (42). They reported significant antibacterial activities for techtochrysin, negletein, and quercetin-3-glucoside; quercetin-3-glucoside was the most potent one, with MIC of 12 μg/mL against B. subtilis, Bacillus cereus, Enterococcus faecalis, Salmonella enteric, S. dysenteriae, and Pseudomonas aeruginosa (42). Quercitin-3-glucoside exhibited potent biofilm inhibition at MIC of 12 μg/mL, with an almost 92-98% reduction in the formation of biofilms in four tested biofilm-forming bacteria (S. aureus, B. subtilis, P. aeruginosa, and E. coli) (42).
In 2015, Madani Mousavi et al. investigated the phytochemical constituents and some biological activities of n-hexane, dichloromethane, and methanol extracts, prepared by the Soxhlet method from the aerial parts of S. platystegia (43). In the mentioned study, dichloromethane and methanol extracts demonstrated antimalarial potentials by inhibition of heme biocrystallization, with IC50 values of 1.19 and 16.94 mg/mL, respectively, without any toxicity at the highest tested concentration (1 mg/mL) in the brine shrimp lethality assay. They also reported the isolation of 2-(4-hydroxy phenyl) ethyl-O-β-D-glucopyranoside from 10% C18-solid phase extraction (SPE) fractions of the methanol extract, as well as apigenin 7-O-glucoside, verbascoside, and martynoside from 40% C18-SPE fractions of methanol extract, using a semi-preparative RP-HPLC analysis (43).
In the present study, a total of 14 phenolic compounds (1 - 14) were isolated from the chloroform and n-butanol fractions of total hydroalcoholic extract obtained from the aerial part of S. platystegia using normal and reversed-phase chromatographic methods on silica gel and Sephadex LH-20 column (Figure 1). Except for verbascoside (compound 7) and apigenin 7-O-glucoside (compound 12), which have been previously reported in S. platystegia, other constituents of this species were detected for the first time.
Flavonoids are one of the largest groups of secondary metabolites identified in the genus Scutellaria (4, 5). These compounds are well-known for their diverse beneficial health effects, such as antioxidant, antimicrobial, anti-inflammatory, anti-aging, and cancer chemopreventive effects (44, 45). Therefore, the isolated flavonoid derivatives can be assumed to be responsible for at least part of the observed antioxidant and antibacterial activities of chloroform and n-butanol fractions, collected from the aerial part of S. platystegia in the current study. Verbascoside (7) and rosmarinic acid (11), two caffeic acid derivatives isolated from this species, are also well-studied bioactive natural compounds, mostly identified in the Lamiaceae family members (46, 47).
In 2016, Ekambaram et al. reported the antibacterial effect of rosmarinic acid against S. aureus and methicillin-resistant S. aureus (MRSA), with MICs of 0.8 and 10 mg/mL, respectively (48). They also showed the synergistic effects of rosmarinic acid with vancomycin, amoxicillin, and ofloxacin against S. aureus, as well as its synergistic effect with vancomycin against MRSA (48). Verbascoside has been also reported as one of the active components of Stachytarpheta indica (Verbenaceae) leaves, with strong growth inhibitory activities against S. aureus and S. epidermis (MIC = 9.77 μg/mL) and moderate antibacterial activities against three Gram-negative bacteria, including Proteus vulgaris (MIC: 312.5 μg/mL), P. aeruginosa, and Acinetobacter baumannii (MIC: 1250 μg/mL) (49).
Moreover, in the present study, the volatile constituents of the aerial part of S. platystegia were analyzed using a GC-MS analysis for the first time. As shown in Table 1, a total of 14 compounds were identified in the essential oil obtained from the aerial part of S. platystegia, with terpinen-4-ol (44.41%), α-terpineol (10.75%), caryophyllene oxide (9.61%), and thymol (8.73%) as its main constituents. Moreover, a review article published by Kasaian et al. on the essential oil composition and biological activities of Scutellaria genus members found hexadecanoic acid, β-caryophyllene, germacrene D, β-farnesene, linalool, and eugenol to be the main constituents of the essential oil of different parts of more than 38 studied Scutellaria taxa (6). Besides intrinsic factors, the observed difference in the essential oil composition of related taxa may be attributed to some developmental and environmental factors (50).
5.1. Conclusions
The present study revealed that S. platystegia has considerable antioxidant and antibacterial effects, as well as a high phenolic content, especially flavonoids and caffeic acid derivatives. The present results suggest this species as a valuable source of potentially active natural compounds in future pharmacological and toxicological studies to develop new herbal antioxidant supplements and novel natural antibiotics.