1. Background
Diabetes mellitus, as one of the most common metabolic diseases worldwide, is known to be more common in middle socioeconomic groups. Approximately 2.5% of the world’s population is diagnosed with diabetes, and this number is estimated to reach 5.4% of the world’s population by 2025 (1). The most important clinical sign of diabetes is high blood sugar, which leads to the glycation and then malfunction of various proteins in the body, causing harmful effects on important organs, such as the heart, kidneys, and eyes (2).
One of the topics discussed in the management of diabetes is the role of oxidative stress and free radicals (3). It seems that oxidative stress and free radicals have adverse effects on sexual organs. The complications of diabetes in the male reproductive system, including erectile dysfunction, testicular shrinkage, sexual reluctance, and changes in semen parameters, have been studied in the literature (4). Progression of diabetes to organs occurs through oxidant activities; research is underway to investigate the use of antioxidants for the treatment of these adverse effects. Moreover, natural antioxidants, as safe, cost-effective, and available options, have attracted the researchers’ attention.
The prostate gland is one of the male accessory sex glands, and in the male reproductive system, it is the most common gland for the growth of malignant tumors. Many compounds in semen, such as fructose, zinc ions, and various proteins, are produced in the prostate (5). In mammals, the prostate gland is formed by many lobes, such as the prostate ventral lobe (6). In this regard, Waters et al. showed that diabetes increased the risk of developing prostate cancer in these patients (7).
Insulin is one of the hormones, which plays an important role in the body metabolism. It is secreted into the bloodstream from the islets of Langerhans, located in the pancreatic endocrine part. Diabetes reduces the body's ability to produce insulin; consequently, diabetic patients need daily insulin injections (8). Insulin therapy is one of the most common treatments for diabetes, which includes restrictions, such as non-oral consumption, short-term effects, and need to be kept in a cool place, which can limit the use of insulin and cause many problems for patients. Considering the shortcomings of insulin injection, researchers are searching for an alternative option from synthetic and plant-based resources (9).
The reactive oxygen species (ROS) molecules are derived from oxygen. An imbalance between the production and elimination of ROS is detrimental in diabetes (10). There are different mechanisms for inhibiting oxidative stress and reducing the toxic effects of ROS. One of these mechanisms is the antioxidant system, which acts as a scavenger of free radicals. Different atypical enzymatic mechanisms involve vitamins A and E, carotenoids, ubiquinones, ascorbic acid, uric acid, taurine, hypotaurine, pyruvate, glutathione, and coenzyme Q, with medical herbs as one of their main sources (11). Vitamin E was introduced in 1992 as the “reproductive vitamin”. It was found that vitamin E was present in spermatozoa and played the role of antioxidants in spermatozoa (12).
Onion or Allium cepa L. belongs to the Liliaceae family. This vegetable has been traditionally consumed as a medical herb. Active compounds of onion include sulfur compounds, such as allyl propyl disulfide. Sulfur compounds are strong antioxidants that act against harmful free radicals (13). Other antioxidant constituents of onion, such as vitamins B₁, B₂, B₆, C, and E, fatty acids, biotin, prostaglandins, pectin, adenosine, and quercetin, have been reported by researchers (14). Onion contains phenol compounds and has many applications in medicine, pharmaceuticals, and food industry among different Allium species (15).
2. Objectives
Complications of diabetes and their impact on the male reproductive system are challenging topics in medicine. Therefore, the present study aimed to investigate the effects of the alcoholic extract of Allium cepa seeds, diabetes (or insulin status), and oxidative stress on sperm parameters and biochemical and histopathological changes of the prostate gland in streptozotocin (STZ)-induced diabetic animals.
3. Methods
3.1. Development of a Laboratory Animal Model of Diabetes
To induce diabetes, following an overnight fast, STZ (Sigma-Aldrich‚ USA) was injected at 60 mg/kg body weight intraperitoneally. By dissolving 10 mg of STZ in 0.1 mL of 0.1 mol citrate buffer, a diabetes induction solution was prepared for rats; the pH of the solution was maintained at nearly 4.5. Almost three days after STZ injection, the rats’ blood glucose levels were evaluated by a glucometer (Beurer GL40‚ Hungary). Animals with blood glucose levels above 250 mg/dL were considered diabetic in this study. The guide for the care and use of laboratory animals (NIH 1985) and all the protocols of Kashan University of Medical Sciences Ethics Committee were adhered to in this study (16).
3.2. Allium cepa L. Seed Extract
Allium cepa seeds were acquired from Barij Essential Pharmaceutical Company (Kashan, Iran). To prepare an alcoholic extract of Allium cepa seeds, 500 g of onion seed was ground to obtain a powder. Next, the powder was mixed with 96% ethanol and left at room temperature for 24 hours. After passing the mixture through a filter and purifying it, it was mixed with 96% ethanol and left for 12 hours; then, it passed through a filter and was purified again. The prepared materials were dehumidified in an oven at 50°C. Finally, the weight of the obtained extract was measured, and solutions of 200 and 400 mg/kg were prepared in distilled water and used for gavage in diabetic rats (17).
3.3. Animals
This laboratory study was performed on 50 adult male Wistar rats (age: 8 weeks, weight: 280 - 320 g), which were kept in a temperature-controlled room in a 12: 12 h light/dark cycle; they had access to a standard rat pellet diet and water ad libitum. The Ethics Committee of Kashan University of Medical Sciences approved this study, based on the Declaration of Helsinki guidelines (1964).
3.4. Insulin Concentration Measurements
Diabetic rats with a fasting blood sugar (FBS) level above 250 mg/mL received insulin daily via subcutaneous injection of 3 U/100 g body weight of insulin (Sigma-Aldrich‚ USA) in the morning (at 10 am, 2 U) and afternoon (at 4 pm, 4 U) for 28 days (18).
3.5. Group Allocation
In this experimental study, 50 rats were randomly selected and divided into five equal groups (n = 10). Group 1 (control) received 0.1 mL of normal saline via oral gavage; group 2 (diabetic rats) did not receive any treatment; group 3 included diabetic rats receiving insulin; and group 4 and group 5 included diabetic rats receiving Allium cepa L. seed extract for 28 days via gastric gavage at doses of 200 and 400 mg/kg (18).
3.6. Tissue Collection and Preparation
After 28 days, the rats were sacrificed under deep anesthesia using chloroform at 24 hours after the final dose of treatment. The animals’ blood samples were collected by measuring the FBS and testosterone levels. The prostate ventral lobe was carefully removed and separated from extra tissues; next, it was weighted accurately using a digital balance. To fix the collected samples for histopathological examinations, they were placed in Bouin’s solution (Sigma-Aldrich, USA). A tissue processor (Shandon-Elliott model) was used to prepare paraffin blocks. Finally, hematoxylin and eosin (H&E) staining was applied to stain the tissue sections (5 μm) (19).
3.7. Epididymal Sperm Collection and Motility Assay
The cauda epididymis was minced with scissors in 1 mL of Ham’s F10 solution and incubated for 20 minutes at 37°C to provide sperms for shedding the epididymis. A drop of the prepared sperm suspension was placed on a slide and studied under a light microscope. To estimate the percentage of motile sperms in each sample, at least 10 microscopic fields were examined at 400X magnification for each sample (20).
3.8. Sperm Viability Assay
To calculate the percentage of live sperms in this study, 20 mL of sperm suspension was mixed with 20 mL of trypan blue and studied under a light microscope (400X magnification). In this method, dead sperms were stained with a dark color. For each sample, at least 200 spermatozoa from 10 fields were counted, and then, the percentage of live spermatozoa was calculated. For each sample, 10 microscopic fields were studied (20).
3.9. Sperm Count Assay
A Neubauer slide was used to count the number of sperms in each sample after diluting the sperm suspension in formalin 10% (20).
3.10. Normal Sperm Morphology
To calculate the number of normal spermatozoa, the sperm suspension was stained with Papanicolaou stain, and the percentage of abnormal spermatozoa was measured using a Neubauer slide (WHO 2010) (20).
3.11. Morphometric Examination of the Prostate Ventral Lobe
Tissue sections of the prostate ventral lobe were studied using an inverted microscope (Nikon Eclipse Ti Inverted Microscope with a DS-L2 camera control unit). In this study, this microscope was equipped with an additional part, called the square lattice, containing 121 intersections to measure the volume density (VD); five tissue sections were examined for each sample.
3.12. Histological Measurement of the Prostate Ventral Lobe
To measure the average diameter of the prostate tubules and lumens, a scale was fitted into an eyepiece, and five tubules were selected randomly in each slide. The average diameter of each tubule was calculated based on the following formula:
The following equation was then used to measure the average diameter of lumens in each tissue section:
In this study, a Nikon microscope was used to evaluate the thickness of the epithelium and fibromuscular tissue in the tubes (21).
3.13. VD Assay of the Prostate Ventral Lobe
In this study, a point-counting technique was applied to evaluate the VD of histopathological changes. VD was measured by dividing the number of points counted in the tissue parameter (PN) by the total number of points counted (Ptn). VD was measured at 100X magnification (21):
3.14. Total Antioxidant Capacity
The total antioxidant capacity (TAC) in the serum was evaluated using the ferric reducing ability of plasma (FRAP) assay. This assay indicates the serum ability to recover iron ions. A blue color is formed when the Fe III-TPTZ complex is converted to Fe II at acidic pH and adsorbed at a maximum wavelength of 593 nm. This procedure requires a significant amount of Fe III. The only determinant of Fe II-TPTZ velocity and blue color production was the vitalizing strength of the samples (22).
3.15. Statistical Analysis
Data were analyzed using ANOVA and Tukey’s post-hoc test in SPSS version 20 (Chicago, IL, USA). A P value less than 0.05 was considered statistically significant.
4. Results
4.1. Blood Chemistry Test Results
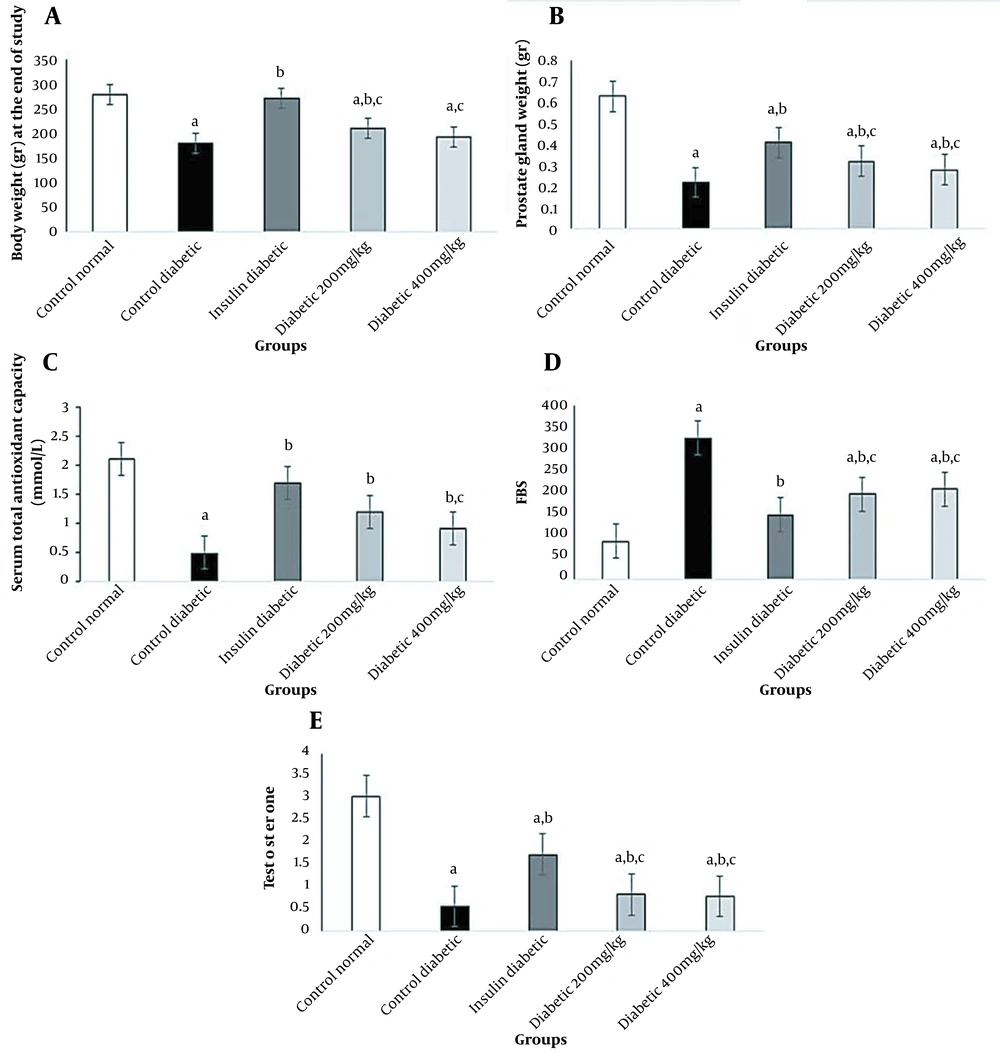
In diabetic rats, the fasting blood sugar (FBS) level increased significantly, while 200 and 400 mg/kg of Allium cepa L. seed extract decreased FBS significantly (P < 0.05). In this study, changes in blood sugar in different groups are shown in Figure 1. Diabetes caused a decrease in the testosterone level in the diabetic group; however, the difference was only significant in the insulin group compared to the control diabetic group (P < 0.05) (Figure 1). The administration of 200 mg/kg of onion seed extract to diabetic animals resulted in a slight increase in the production of testosterone compared to the control diabetic rats (P < 0.05).
Effects of onion seed extract and insulin on the body and prostate weight at the end of the study, total antioxidant capacity (TAC), blood sugar, and testosterone level in diabetic rats. Values are expressed as mean ± SD in each group (significant difference compared to the control group at P < 0.05).
The results of this study demonstrated that serum TAC levels significantly reduced in the diabetic rat group compared to the normal control group (P < 0.05). The consumption of onion seed extract at 200 mg/kg caused a significant increase in TAC compared to the diabetic control group; however, at a dose of 400 mg/kg, the difference was significant compared to the insulin group (P < 0.05) (Figure 1).
Figure 1 compares the effects of STZ-induced diabetes, administration of Allium cepa L. seed extract, and insulin injection on the body weight and prostate weight. The results revealed that the consumption of Allium cepa L. seed extracts at 200 and 400 mg/kg could preserve the prostate weight significantly similar to the insulin group; however, in the oxidative stress group, the body and prostate weights significantly reduced (P < 0.05).
4.2. Sperm Analysis
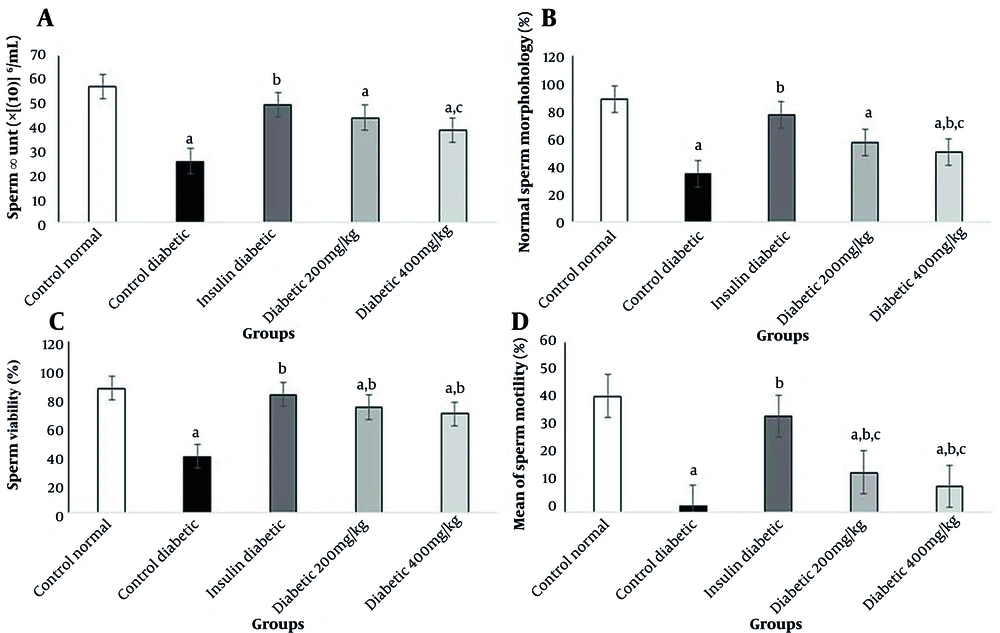
The present results indicated a significant reduction in the number of motile sperms in diabetic rats. Treatment with insulin in group 3 caused a significant increase in the motility of sperms. Also, administration of Allium cepa L. at 200 and 400 mg/kg caused a significant increase in progressive sperms compared to STZ-treated rats (P < 0.05). The results showed a significant decrease in sperm viability in diabetic rats (P < 0.05). Also, sperm viability increased significantly in the insulin group compared to the STZ-treated rats (P < 0.05). The injection of insulin could increase sperm count significantly compared to STZ-treated rats (P < 0.05); this finding shows that sperm health is directly related to the amount of blood insulin. Other changes in sperm parameters are presented in Figure 2.
4.3. Histopathological Changes
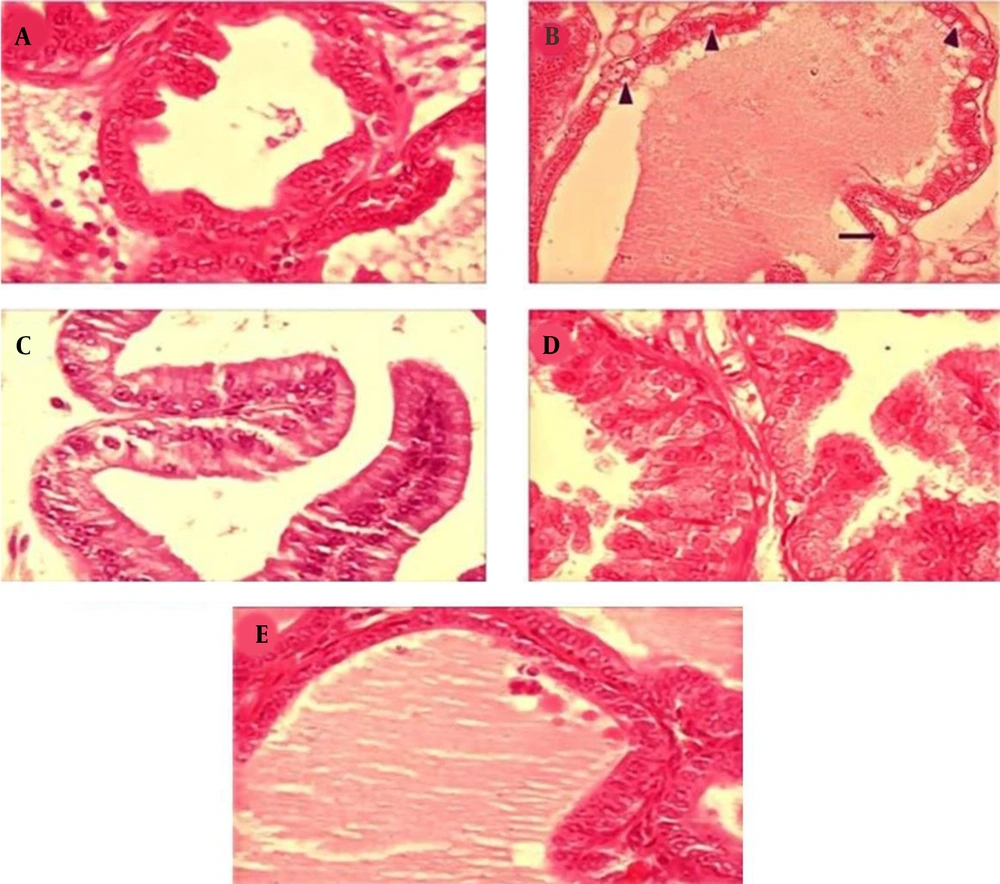
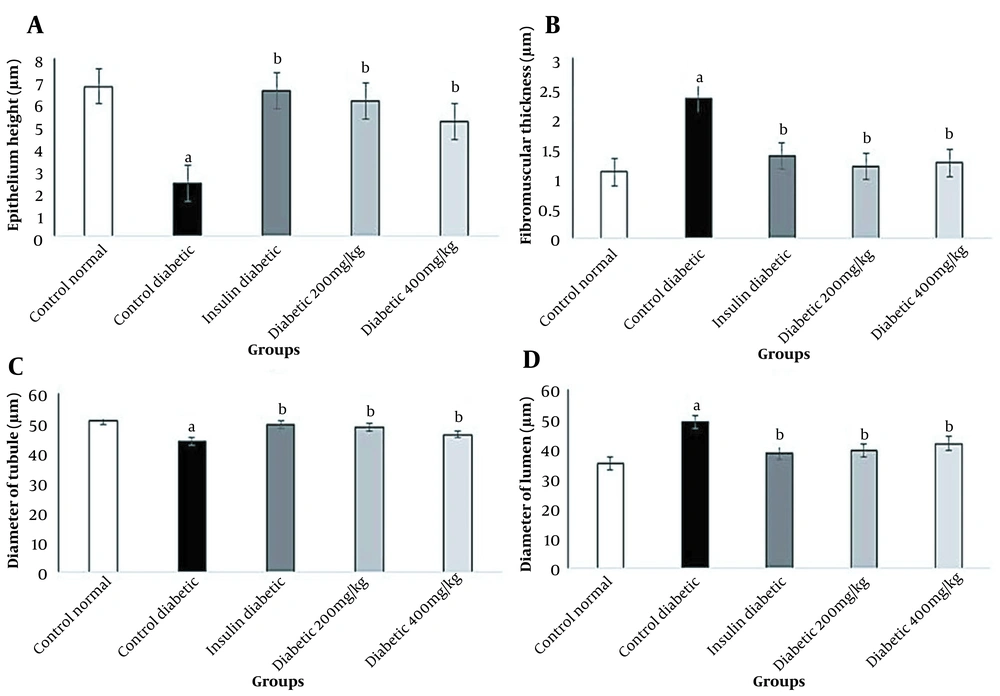
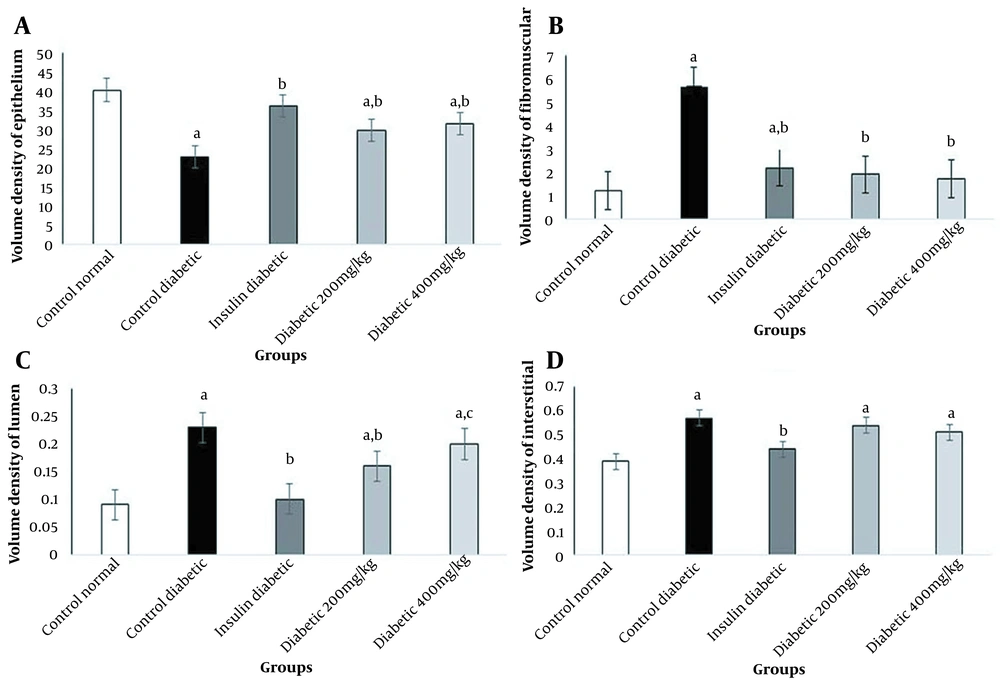
In this study, diabetes caused histopathological changes in rats receiving STZ. Figure 3 demonstrates changes, such as decreased epithelial diameter and increased fibromuscular tissue diameter in diabetic rats. The mean values of the epithelium diameter, fibromuscular layer thickness, tubule diameter, lumen diameter, and VD in the prostate ventral lobe are shown in Figures 4 and 5. In insulin-treated rats, the epithelium thickness increased significantly, while the fibromuscular thickness decreased significantly (P < 0.05). Changes caused by diabetes improved significantly by consumption of onion seed extracts. Onions at 200 and 400 mg/kg not only significantly increased the epithelium height, but also partially decreased the fibromuscular thickness (P < 0.05). Other histopathological changes are presented in Figures 4 and 5.
Effects of Allium cepa seed extract on the histology of the prostate ventral lobe in STZ diabetic rats. A, The normal epithelium and luminal content of the control group; B, Adverse effect of STZ treatment on the prostate tissue, such as an increase in interstitial volume‚ fibromuscular volume‚ vacuolization (▲)‚ and separation of tissue (→) in diabetic rats; C, Effects of insulin therapy on the prostate; in the insulin group, the recovery of the prostate epithelium can be observed; D and E, the prostate tissue recovery by administration of Allium cepa seed extract at 200 and 400 mg/kg. Sections were accumulated and dyed with H&E and observed under 400x magnification.
Effects of onion seed extract administration on the volume density (VD) in the prostate ventral lobe. Values are expressed as mean ± SEM in each group. A, significant difference compared to the control group at P < 0.05; B, significant difference compared to the control group at P < 0.05; C, significant difference compared to the insulin group at P < 0.05.
5. Discussion
Many studies have used medicinal plants to treat and prevent the complications of diabetes. The results of this study indicated the effects of onion seed alcoholic extract on the blood chemistry, sperm parameters, and histopathological changes in the prostate ventral lobe of STZ-induced diabetic rats. Sperm parameters in the diabetic group showed a significant decrease compared to the control group. On the other hand, a significant increase was observed in the sperm motility of the diabetic group treated with Allium cepa L. at 200 mg/kg compared to the diabetic group. The findings of several previous studies are consistent with the current results. In this regard, Aghaei et al. demonstrated that in cyclophosphamide-treated rats, histopathological changes in the epididymis, such as vacuolization, disorganization, and separation of the epididymal epithelium, as well as sperm parameters, ameliorated remarkably by consumption of pumpkin seed extract (20). Moreover, Jalili et al. found that administration of Falcaria vulgaris extract could increase sperm parameters, such as sperm motility, count, and viability; it could also ameliorate histological changes of the epididymis in diabetic rats (23).
Spermatogenesis is the process of sperm production in males, which is influenced by various factors. One of these factors is oxidative stress, caused by the accumulation of ROS. In diabetes, the balance between oxidative stress and antioxidants is disturbed, causing irreversible side effects, such as decreased fertility or inability to reproduce in diabetic patients (24). ROS can interfere with RNA production and mitochondrial function in spermatozoa (25). Salahshoor et al., in 2018, showed that hyperglycemia in diabetic rats could significantly reduce sperm parameters, which is in line with the results of this study (26). There are many plants with a high antioxidant content that can improve the sperm motility, as well as the morphological characteristics of sperms. In this regard, Asadi et al. demonstrated that antioxidants could protect sperms against free radical damage (27). It seems that the antioxidant resources of onion could improve some sperm parameters similar to insulin injection in this study.
The present study indicated that Allium cepa L. seed extracts, administered at doses of 200 and 400 mg/kg, could improve histological changes, such as the epithelial diameter. These histopathological changes may be due to the presence of several antioxidants, such as quercetin‚ selenium, glutathione, and vitamins. The findings of the current study are in agreement with those reported by Ola-Mudathir et al., which indicated that onion had significant effects on decreasing oxidative stress in the rat testes (28). Besides, Nikravesh et al. indicated that the consumption of onion in mice could ameliorate the histological changes of testis (14). These protective effects of Allium cepa L. seed extract are possibly due to its high content of antioxidants, such as flavonoids (quercetin and isorhamnetin), selenium, glutathione, and vitamins A, B, and C (17).
Additionally, Khaki et al., examining the effects of onion on the reproductive power of rats after using an antiepileptic drug (lamotrigine), found that onion antioxidants, such as different vitamin groups, are beneficial for ameliorating the toxic effects of free radicals (29). The current results demonstrated that oxidative stress had a decreasing effect on the body and prostate weights. Oxidative stress in diabetic rats could induce histopathological changes, such as vacuoles and shrinkage of the epithelium. These findings are in line with the results of a study by Kamel and Abd-Elrhman, which showed that diabetes was associated with prostate complications in diabetic rats (30).
This study also showed that reduction of FBS due to the consumption of Allium cepa L. seed extract at 200 and 400 mg/kg was significant. The results of the present study are consistent with the findings of a study by Kang et al., which investigated the effect of onion on diabetes mellitus and reported that an onion diet could decrease FBS significantly (31). Moreover, Taj Eldin et al. investigated the clinical hypoglycemic effects of Allium cepa L. in diabetic patients and reported that consumption of crude Allium cepa led to a remarkable reduction of FBS in type 1 diabetic patients (32). Also, Kim et al. demonstrated that quercetin is a major phenolic compound in onion extracts with a significant α-glucosidase inhibitory activity (33). In this study, insulin therapy increased histological changes and sperm parameters substantially. The results of this study are similar to the findings of a study by Bahey et al., which revealed that insulin therapy improved the histological changes of prostate in diabetic rats (34). Patel et al. also reported that an antioxidant compound in Allium cepa, called garden cress which may be found in medical herbs, could increase the blood insulin level in diabetic rats (35).
The levels of testosterone decreased significantly in diabetic rats compared to the normal controls, while in the insulin group, testosterone showed a significant increase compared to the diabetic group (Figure 5). In this study, consumption of onion extracts at 200 and 400 mg/kg caused a significant increase in testosterone levels compared to the control diabetic group; this increase was significant compared to the effect of insulin. The current results are in agreement with the results of a study by Khaki et al., which demonstrated that daily consumption of fresh onion extract (at doses of 0.5 g per rat and 1 g per rat) for 20 days caused a significant increase in the testosterone levels of rats (36). The findings showed that oxidative stress in diabetes led to a significant reduction in TAC compared to the normal control group. Based on the results, the consumption of onion seed extract at 200 and 400 mg/kg caused a significant increase in TAC compared to the diabetic control group. The effects of decreased TAC in the diabetic group caused tissue damage in the prostate gland. Overall, diabetes causes oxidative stress in the gonads, which manifests itself as increased ROS and lipid peroxidation and decreased TAC (37). In this study, antioxidants in onions reduced oxidative stress in the prostate gland.
5.1. Conclusions
The results of the present study demonstrated that the onion seed extract could ameliorate STZ-induced complications in the prostate ventral lobe and improve sperm parameters in diabetic rats. Based on the results, it can be proposed that the use of onion seed extract, together with insulin therapy, has potential protective effects on diabetes mellitus. The findings of this study suggest that onion seeds, as a suitable source of complementary foods rich in antioxidants, has beneficial effects on diabetes. Further studies are needed to clarify the molecular mechanism responsible for the increasing effect of onion extract on sperm parameters.